Christian Lautenschlager, PhD and Ryan Hamnett, PhD | 15th March 2024
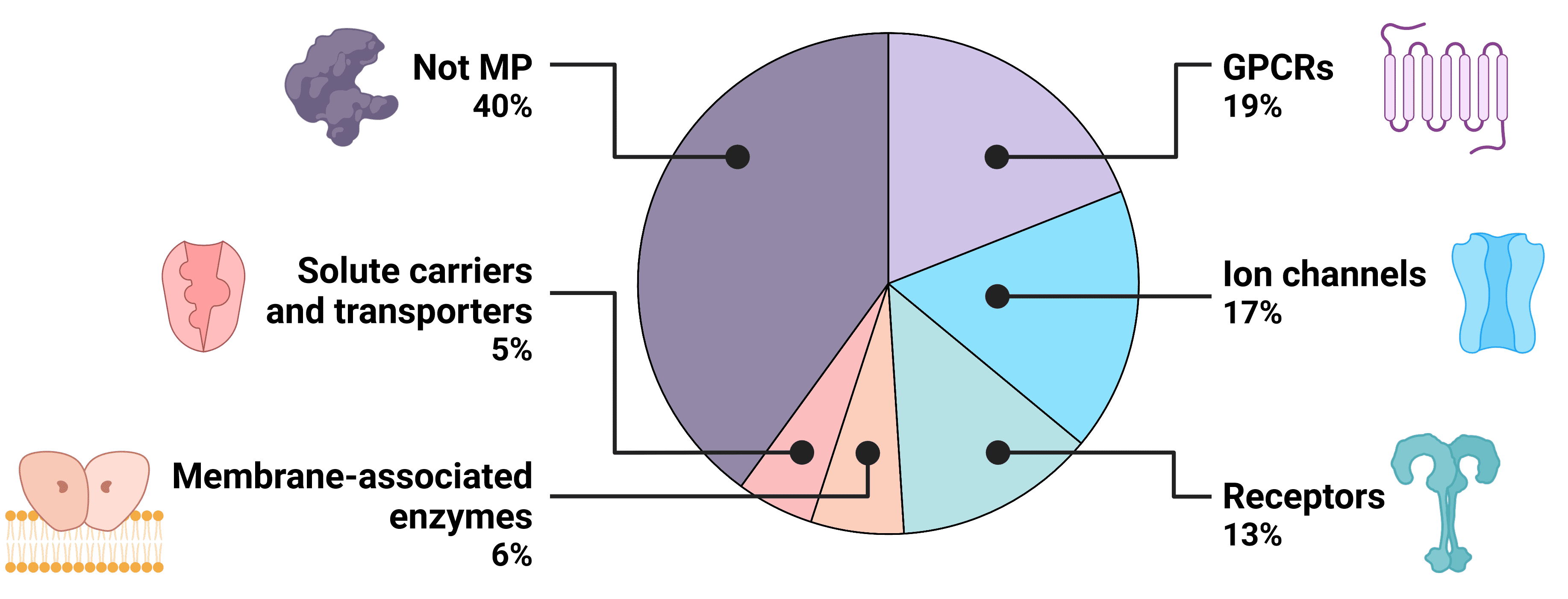
Transmembrane proteins (TMPs) span the lipid bilayer of living cells, acting as bridges across which the external environment can regulate cellular activity. Transmembrane proteins are involved in diverse cellular functions, including material transport, signal transmission, and cell-cell recognition, making them prominent targets for drug development to modulate cellular processes or target specific cell types. Though transmembrane proteins make up just 23% of the human proteome, over 60% of known drugs now target transmembrane proteins1,2, including BCMA, GPRC5D, and CCR8, for the treatment of conditions such as cancer, cardiovascular disorders, neurological conditions and autoimmune disorders.
Figure 1: The druggable human proteome.
However, the isolation and study of transmembrane proteins, especially multi-pass transmembrane proteins with multiple hydrophobic transmembrane regions, can be technically demanding. In contrast to single-pass transmembrane proteins, which can typically be studied via their extracellular domain (ECD), full-length multi-pass transmembrane proteins must be used. Hydrophobic regions make them susceptible to in vitro aggregation, while their three-dimensional structure and conformation, essential to maintain for functional assays and structural biology, are sensitive to environmental conditions.
Through technical innovation within this complex landscape, we provide a suite of research tools to enable you to study transmembrane proteins and generate unprecedented insights into transmembrane protein structure, function, and interactions.
Nanodiscs are nanoscale structures (10-50 nm) comprising transmembrane proteins within a phospholipid bilayer, held by a stabilising belt. First conceived by the laboratory of Professor Stephen G. Sligar in 20023, nanodiscs offer a stable and biologically relevant environment to enable the isolation of TMPs in their native conformation.
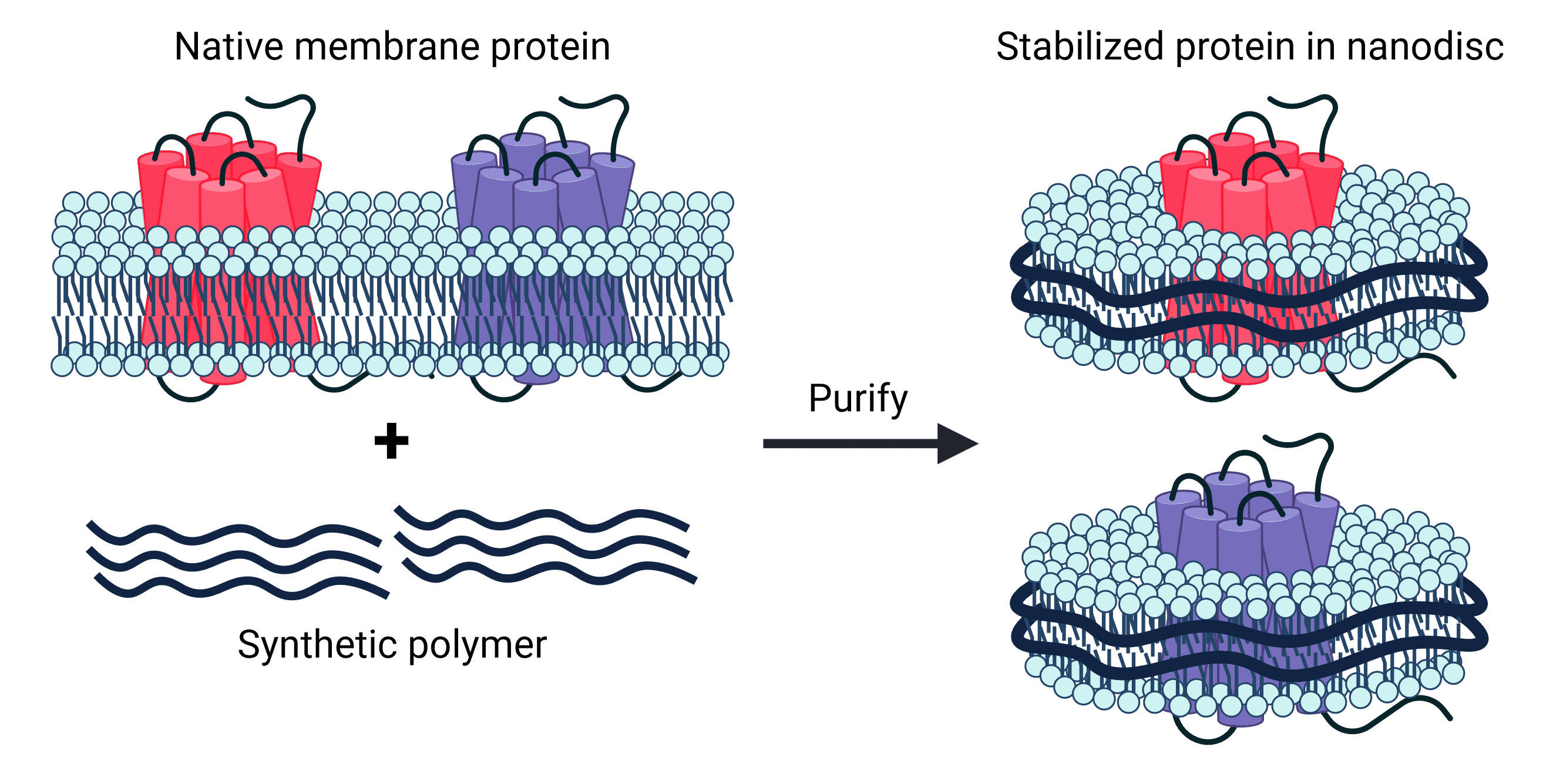
Synthetic nanodiscs, one of the two types of nanodisc, are composed of native cell membrane phospholipids encircled by belts made from a variety of synthetic polymers, such as styrene-maleic acid co-polymers (SMAs) or Diisobutylene-maleic acid (DIBMA), offering great control and versatility in application4,5. These synthetic belts have been engineered for stability and solubility, meaning no detergents are required in the isolation of transmembrane proteins held within the nanodiscs. Furthermore, nanodisc proteins are produced in mammalian cell expression systems, which ensure proper folding and post-translational modifications (PTMs) of proteins. This is particularly important for complex multi-pass transmembrane proteins, which often require specific chaperones and co-factors for assembly.
Learn more about nanodiscs on our Nanodiscs page
Figure 2: Synthetic nanodiscs containing full-length TMPs in a phospholipid bilayer are generated from native cell membranes.
Advantages of Nanodiscs:
Nanodisc Applications:
Figure 3: Synthetic Nanodisc Human TLR4 Protein (A318415) can bind Anti-Claudin18.2 Chimeric Antibody [Zolbetuximab Biosimilar] - Azide free (A318887) with an affinity constant of 1.619 nM as determined in a SPR assay.
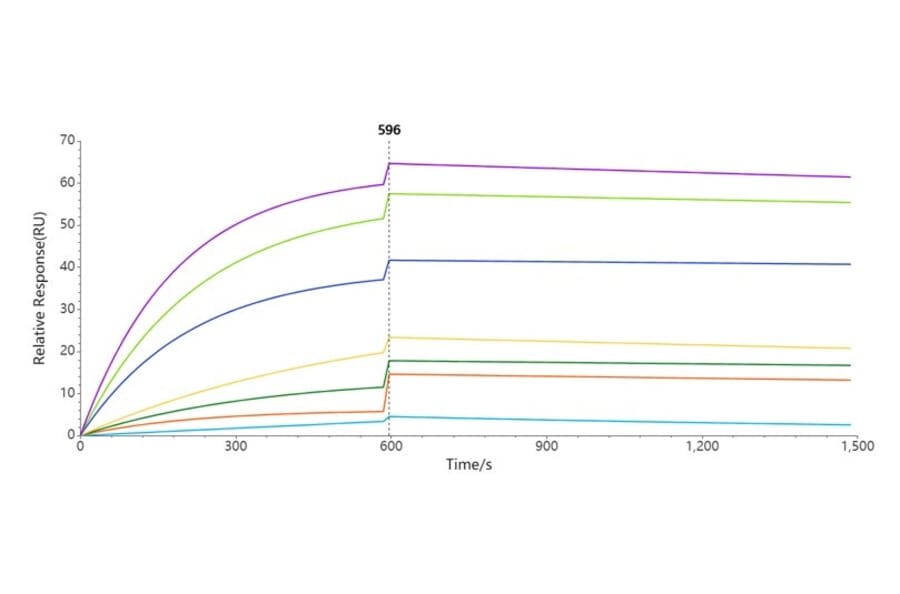
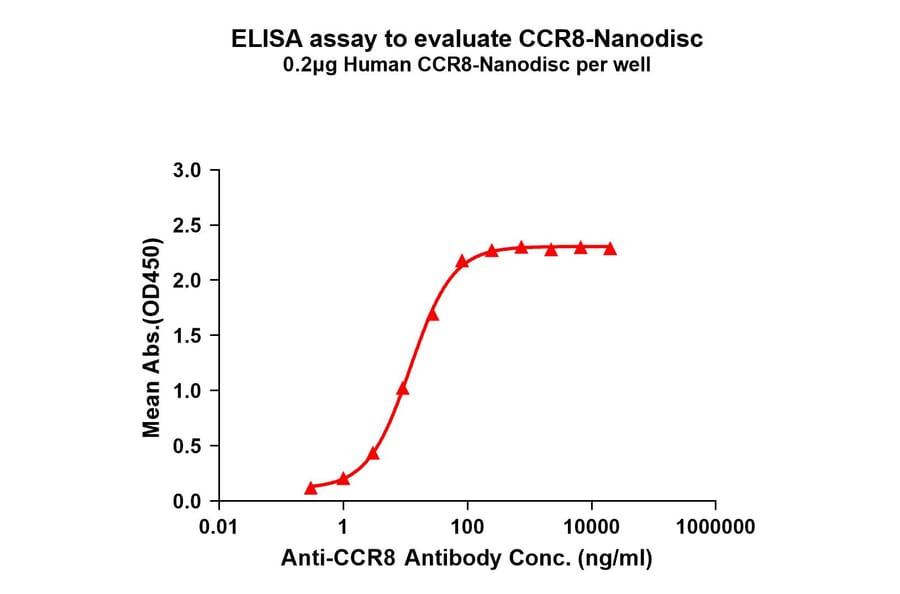
Figure 4: Immobilized Synthetic Nanodisc Human CCR8 Protein (A318461) binds Anti-CCR8 Humanized Antibody [BMS 986340] - Azide free (A318852). The EC50 for this binding is 12.07 µg/ml.
Virus-like particles (VLPs) are non-infectious structures that mimic the shape of viruses but lack a viral genome. During VLP production in cells or cell-free systems, VLPs self-assemble into 100-150 nm protein particles, and incorporate transmembrane proteins into a VLP-MP complex, resulting in presentation of the protein on the external surface of the VLP.
Figure 5: VLP-TMP complexes are generated containing full-length transmembrane proteins following self-assembly.
Advantages of VLPs:
VLP Applications:
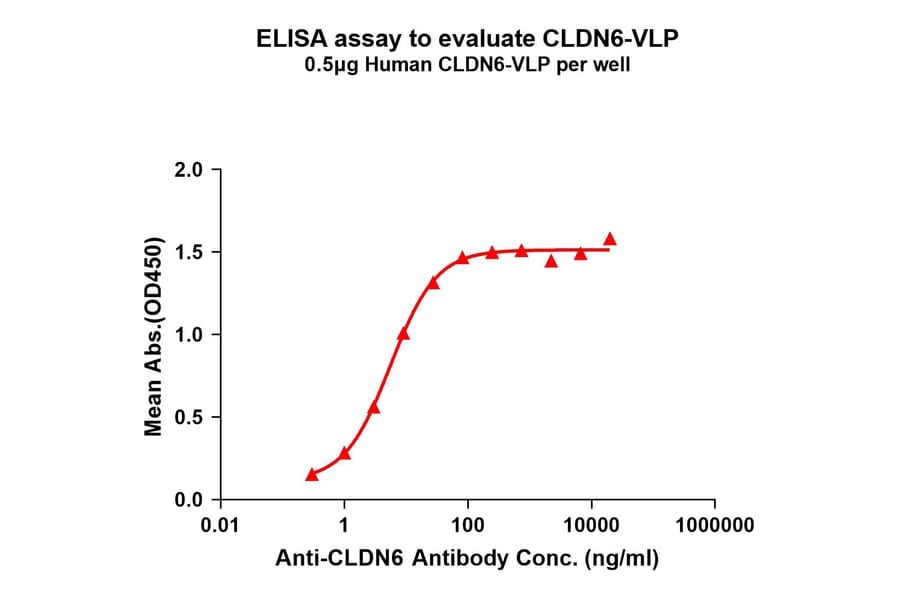
Figure 6: Immobilized Synthetic Virus-like Particle Human Claudin 6 Protein (A318460) binds Anti-Claudin 6 Humanized Antibody [IMAB027] - Azide free (A318880). The EC50 for this binding is 5.616 µg/ml.
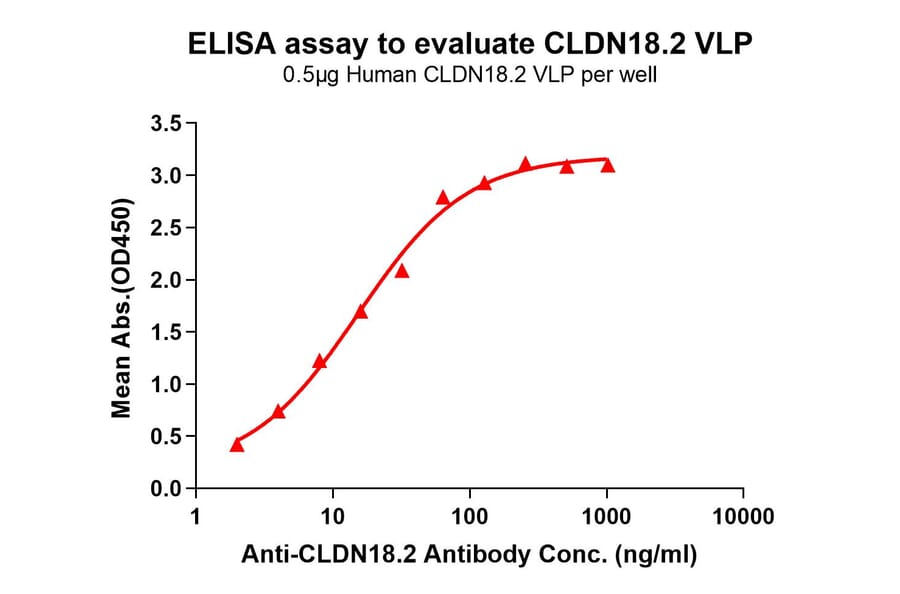
Figure 7: Immobilized Synthetic Virus-like Particle Human Claudin18.2 Protein (A318490) binds Anti-Claudin18.2 Chimeric Antibody [Zolbetuximab Biosimilar] - Azide free (A318887). The EC50 for this binding is 15.37 µg/ml.
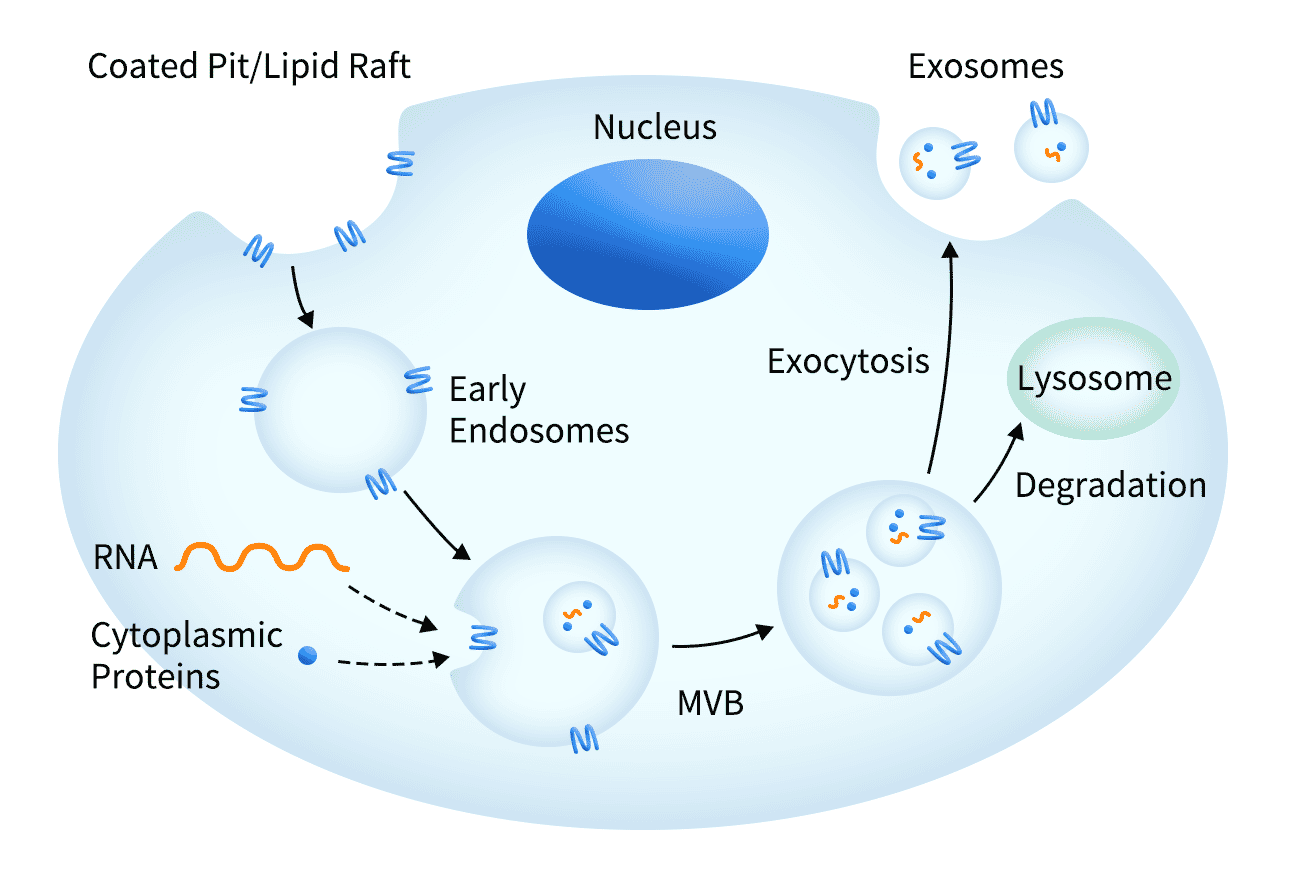
Exosomes are small (30-150 nm) membrane-bound vesicles secreted by the cell, which mediate cell-cell communication and play an important role in the immune response. Each vesicle contains a variety of molecules including proteins, RNA and DNA.
Exosomes are useful for supporting transmembrane protein molecules by providing an optimal environment to maintain receptor solubility. The transmembrane proteins are overexpressed, localised to the host cell membrane, and subsequently secreted within exosomes, which are purified for downstream applications. As a result of the exosome being secreted, there is no need to perform harsh membrane extraction techniques which is sometimes necessary with traditional techniques.
Figure 8: Exosomes are naturally formed and secreted by cells, containing transmembrane proteins in the cell membrane as well as other biological molecules.
Advantages of Exosomes:
Exosome Applications:
| Popular Exosome Transmembrane Proteins | |
|---|---|
| Synthetic Exosome Human CD24 Protein (A318492) | Synthetic Exosome Human GPCR GPRC5D Protein (A318487) |
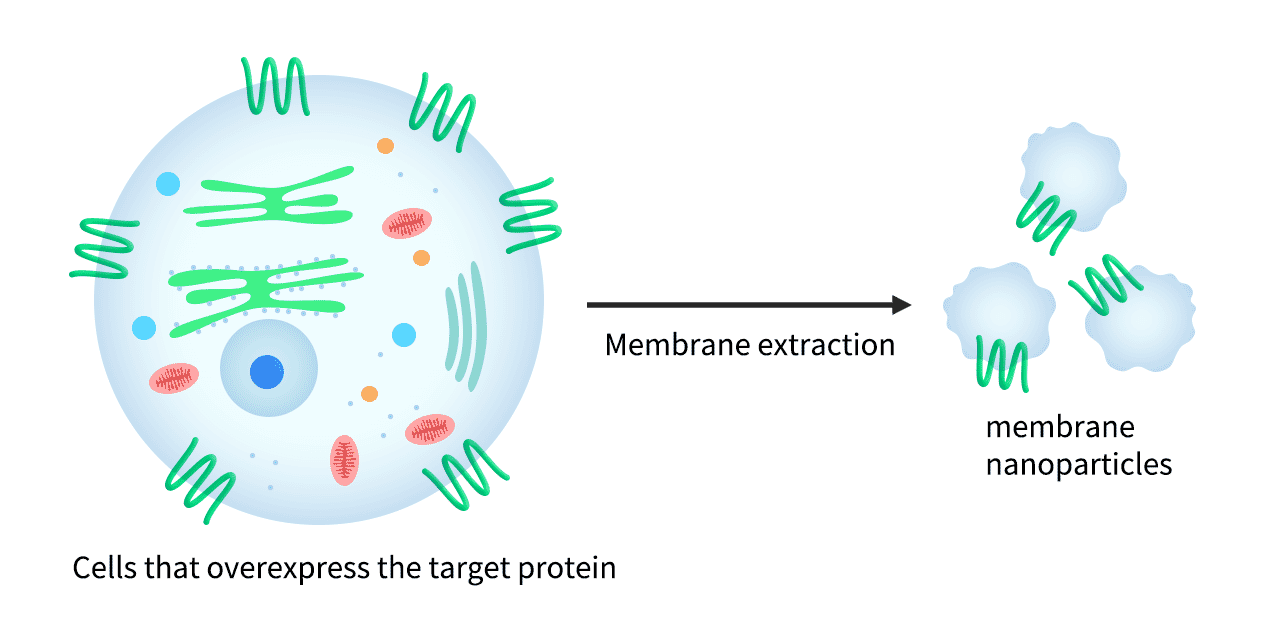
Membrane nanoparticles (MNPs) comprise a synthetic nanoparticle coated with a layer of natural cell membrane. Membrane nanoparticles are extremely useful for isolating transmembrane proteins because of their small size (10-100 nm) and because the lipid bilayer maintains MP stability and solubility.
To form MNP-TMP complexes, the transmembrane protein is expressed within HEK293 cells and displayed on the cell surface, before proprietary extraction processes are used to isolate the membrane from the host cells and produce the target protein-containing membrane nanoparticles.
Figure 9: Overexpressed TMPs within the cell membrane are extracted and packaged as membrane nanoparticles.
Advantages of Membrane Nanoparticles (MNPs):
Membrane Nanoparticles (MNPs) Applications:
Extracellular domain (ECD) proteins refers to the region of an transmembrane protein located on the outside of the cell. Many drugs against transmembrane proteins target the ECD, given their exposure and role in interacting with other cells and signalling molecules. We provide over 1,000 purified extracellular domain proteins, made using the HEK293 mammalian cell expression system. To ensure the highest quality standards, we assess purity, antibody-drug interaction, and stability testing.
Advantages of Extracellular Domain (ECD) Proteins:
Extracellular Domain (ECD) Proteins Applications:
Biosimilar antibodies are designed to mimic existing, FDA approved therapeutic antibodies, but are manufactured for research use only (RUO). They have a wide range of applications in biological research, including as standards to compare new lead candidates against for binding affinity, association and dissociation. In the study of TMPs, biosimilar antibodies can be used for functional characterisation of a target TMP.
Advantages of Biosimilar Antibodies
Biosimilar Antibody Applications:
Figure 10: ELISA plate pre-coated by 2 µg/ml (100 µl/well) Recombinant Human PD-L1 Protein (Fc Chimera 6xHis Tag) (A318385) can bind Anti-PD-L1 Humanized Antibody [Atezolizumab Biosimilar] - Azide free (A318947) in a linear range of 0.24-7.81 µg/ml.
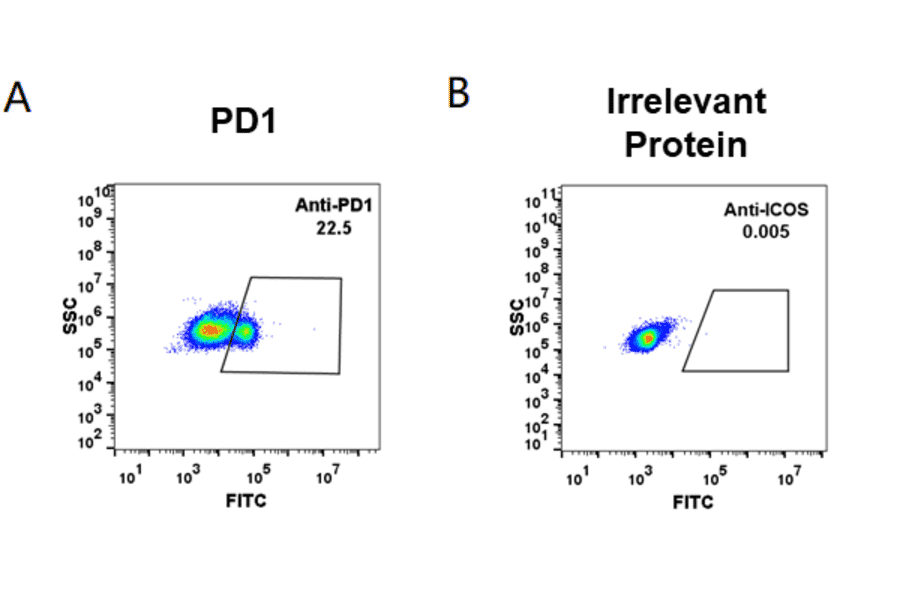
Figure 11: HEK293 cell line transfected with human PD1 (A) and irrelevant protein (B) were surface stained with Anti-PD1 Humanized Antibody [Pembrolizumab Biosimilar] - Azide free (A318950) at 1 µg/ml followed by Anti-Human IgG Antibody (Alexa Fluor™ 488™).
| Popular Biosimilar Antibodies | Validated Applications |
|---|---|
| Anti-CD52 Humanized Antibody [Alemtuzumab Biosimilar] (A318926) | Flow Cytometry |
| Anti-CD22 Humanized Antibody [Pinatuzumab Biosimilar] (A318927) | ELISA, Flow Cytometry |
| Anti-DR5 Humanized Antibody [Tigatuzumab Biosimilar] (A318924) | ELISA, Flow Cytometry |
| Anti-PD1 Humanized Antibody [Pembrolizumab Biosimilar] (A318950) | ELISA, Flow Cytometry |
| Anti-PD-L1 Humanized Antibody [Durvalumab Biosimilar] (A318821) | ELISA, Flow Cytometry |
| Anti-PD-L1 Humanized Antibody [Atezolizumab Biosimilar] (A318947) | ELISA, Flow Cytometry |
| Anti-TIGIT Humanized Antibody [Vibostolimab Biosimilar] (A318929) | ELISA, Flow Cytometry |
| Anti-TWEAKR Humanized Antibody [Enavatuzumab Biosimilar] (A318925) | ELISA, Flow Cytometry |

![ELISA - Anti-PD-L1 Humanized Antibody [Atezolizumab Biosimilar] - Azide free (A318947) - Antibodies.com](https://cdn.antibodies.com/image/catalog/318/A318947_1.png?profile=product_image)