Lucas Baumard, PhD | 14th April 2025
Epithelial cells are one of the most abundant cell types throughout the body, covering the skin, lining blood vessels (see Endothelial Cell Markers) as well as the respiratory, genital-urinary and digestive tracts.
Epithelial cells are found throughout the body and are highly specialized by function, shape and orientation, to their tissue and niche. Therefore, epithelial cell markers differ based on location, tissue and differentiation state, with the re-emergence of stem cell-like markers in differentiated epithelial cells being a hallmark of tumorigenesis.
Epithelial cells are classically characterized by their shape and how they organize (Figure 1): squamous, cuboidal, columnar, and simple, stratified, transitional, respectively. Most epithelial cells throughout the body act as barrier cells, secretory cells or regulate absorption.
Figure 1: Epithelial cell morphologies and functions. Reproduced under Creative Commons 4.0 CC-BY from Ambrogi, M & Vezina, CM. Roles of airway and intestinal epithelia in responding to pathogens and maintaining tissue homeostasis. Front. Cell. Infect. Microbiol. 14 (2024).
In their roles as barrier and secretory cells, epithelial cells possess several distinct polarities: the apical aspect faces the lumen or externally; the basal aspect connects to the basal lamina; the luminal aspect links neighboring cells. Because of this, and combined with their numerous niches and tissues of residence, epithelial cells possess a large number of varied cell surface markers whose expression can vary with, and be directly linked to, diseases like cancer, epithelial mesenchymal transition, fibrosis, asthma, COPD, Crohn’s and celiac disease.
The wide range of diseases and clinical conditions associated with epithelial dysfunction reflects their diverse roles throughout the body. For instance, epithelial cells within the respiratory tract feature ciliated and secretory cells and play a pivotal role in maintaining mucociliary clearance.23 Their dysregulation is associated with chronic obstructive pulmonary disease (COPD) and asthma.24 Meanwhile, epithelial cells within the mammary glands fulfil several different roles, from luminal, cuboidal epithelial cells that form milk-producing structures, to myoepithelial cells which provide shape and support.25 BRCA1-associated breast cancers have been linked to the progenitors of these luminal epithelial cells.26
Epithelial cells share common features despite a varied origin and function. Epithelial cells most often are bound to either each other (adherens junctions, tight junctions, desmosomes1) or a basement membrane, (trans-membrane integrin heterodimers2). Examples of some of these more common epithelial cell markers include:
Figure 2: IHC of human Intestine stained with Anti-Claudin 3 Antibody [ABT-CLD3] (A97074).
Figure 3: Flow cytometry analysis of MCF-7 human breast adenocarcinoma cell line with Anti-Cytokeratin 19 Antibody [A53-B/A2] (FITC) (A86359).
Epithelial Lineage Markers
During embryogenesis and development, epithelial cells can derive either from the ectoderm, mesoderm, or endoderm 1. A brief summary of these germ layers and their common markers can be found in Table 1.
| Germ Layer | Mature Tissues | Markers | References |
|---|---|---|---|
| Endoderm | Digestive and respiratory tract | CXCR4, KIT, EPCAM, SOX17, GATA4 | 3-6 |
| Ectoderm | Brain, spinal cord, nerves, skin | NES, PAX6 | 7,8 |
| Mesoderm | Bone, cartilage, muscles | NCAM1, TBXT | 9,10 |
Table 1: Epithelial developmental lineage markers
After embryogenesis, the development and maintenance of epithelial cells and tissues differs drastically depending on their location and function.
Epithelial Markers in the Digestive Tract
In the esophagus, multiple basal cell layers (not stem cells) give rise to various differentiated basal epithelial sub types before reaching the fully differentiated luminal-facing epithelial cell.16 Research has shown that the activity of bone morphogenetic proteins (BMPs) on BMP receptors promotes normal squamous differentiation of basal progenitor cells in the adult esophagus, whereas BMP inhibition drives eosinophilic esophagitis.17
Throughout the colon and small intestine, the stem cells that give rise to the epithelium are located in the base of regions called crypts. As the cells move up the crypt, they cease proliferation and begin differentiation into the different cell lineages of the mature villi: enteroendocrine cells and goblet cells, for example.1 The loss of E-cadherin function here has been shown to promote epithelial-mesenchymal transition (EMT),11 and the switch to N-cadherin is associated with tumorigenesis.12
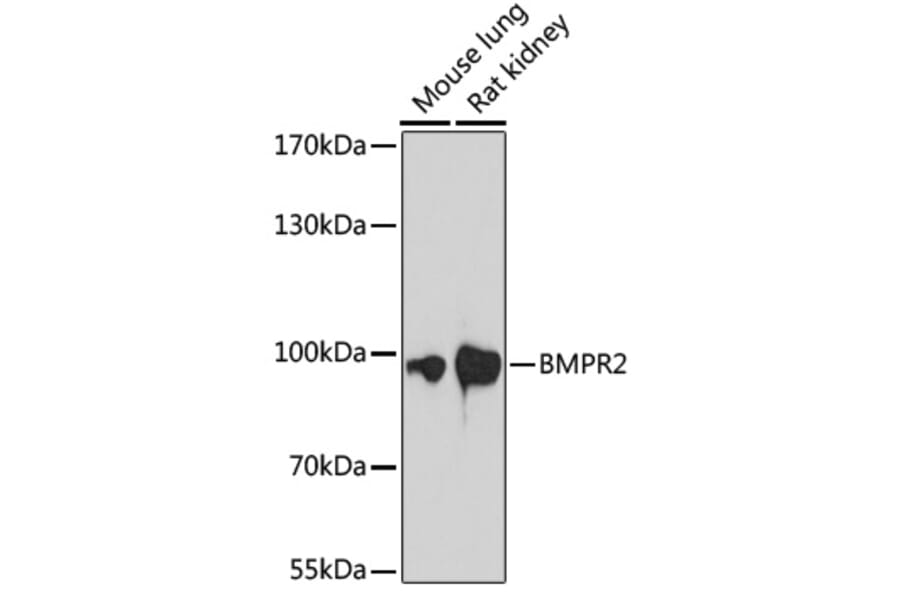
Figure 4: Western blot of various lysates using Anti-BMPR2 Antibody (A87724).
Figure 5: IHC of human heart with Anti-N Cadherin Antibody [MNCD2] (A248060).
Epithelial-Mesenchymal Transition (EMT)
EMT is the process by which epithelial cells lose their typically close adhesion to nearby cells and adopt the migratory and invasive phenotype of mesenchymal cells instead, enhancing their production of extra-cellular matrix (ECM) components and gaining resistance to apoptosis. EMT is a necessary biological process during embryonic and organ development, but is also associated with cancer and metastasis.
A hallmark of EMT is the movement of cells from the basement membrane through the ECM. Changes to cell-ECM interactions can be reflected in changing expression of the Integrin family of transmembrane receptors.13 In other situations, integrins can drive EMT. The alphav beta6 integrin (normally associated with wound healing and embryogenesis) has been shown to enhance tumorigenesis through autocrine release of TGF-β (another important EMT factor)14 and increased migration.15
Carcinoembryonic Antigen (CEA) in Developing and Mature Epithelium
Carcinoembryonic antigen (CEA) is an oncofetal antigen: it is uncommonly expressed after fetal development yet is highly expressed in a some cancers.18 CEA is expressed on only a few, specific epithelial cells within healthy adult tissue, making it a useful marker for these cells (Table 1).
| Tissue | Cell Types | Reference |
|---|---|---|
| Colon | Columnar epithelial cells, Goblet cells | 19-21 |
| Stomach | Mucous neck cells, Pyloric mucous cells | 22 |
| Tongue, esophagus, cervix | Squamous epithelial cells | 20 |
| Pancreas | Secretory epithelia, Duct cells of sweat glands | 21 |
Table 2: CEA expression in different epithelial subtypes
Epithelial cells form 2D sheets and 3D layers depending on tissue type and are therefore in contact with each other as well as neighboring cell types (Figure 6). In their role as barriers, the expression of binding proteins on the luminal surfaces of epithelial cells regulates the rigidity and porosity of the barrier. In turn this can regulate absorption and secretion and prevent the ingress of pathogens. Some of these markers are downregulated in various tumors, which can help prevent immune cell integration and killing.27
Figure 6: Epithelial cell junction proteins.
Of these adhesion markers, epithelial cell adhesion molecule (EpCAM or TACST-1, TROP1, CD326) is one of the most well studied, being expressed in most epithelial cell types throughout the body and over expressed in an equal number of cancers in various tissues.28 This transmembrane glycoprotein mediates cell-cell adhesion but is structurally different to the other adhesion protein families.29
Claudins are a moderately large (24 member) family of tight-junction, transmembrane proteins widely expressed on epithelial cells.30 They are important proteins in the regulation of intra-cellular transport.31 Mutations in the claudin genes are associated with metabolic and mineral-dependent diseases such as kidney failure and magnesium wasting disease.32 Their expression is highly upregulated in some cancers, whilst down regulated in others.33
Figure 7: Immunofluorescence of MCF-7 cells using Anti-EpCAM Antibody [EGP40/837] (A249262) in green. Nuclei are stained with NucSpot in red.
Figure 8: IHC of human colon with Anti-Claudin 4 Antibody [ABT-CLD4] (A97073).
Epithelial cell proteins can also provide structural support for either their host cell or neighboring cells.
The keratin (or cytokeratin) family are filament forming proteins that make up the main cytoskeletal component of epithelial cells and provide mechanical support. They are broadly divided into type I and type II (Ka- and Kb- prefixes, respectively), with epithelial keratins including both types.34 There are 54 members across these two types. Though mostly associated with epithelial cells, they are also expressed on some endothelial35 and muscle cell types.36
Mutations in the keratins genes are linked to specific tissue-fragility disorders and cancer,35 with antibodies against the proteins being a useful tool in diagnostic pathology. Keratins are also associated with various tumor types:29 overexpression of keratin-19,37 and the loss of keratin-8 and -18, are markers of epithelial mesenchymal transition.38 Antibodies against keratin can be found against individual keratin proteins or against several keratin proteins. The antibodies MOC31 and CK AE1/AE3 are commonly used to diagnose a positive cancer state by the positive presence of keratins.
Epithelial membrane antigen (or MUC1), is a glycoprotein expressed on the apical surface of glandular epithelial cells, where it maintains a physically barrier and prevents adhesion of pathogens.39 It is also able to bind growth factors such as connective tissue growth factor, platelet-derived growth factor A and B and epidermal growth factor.40 Overactivation of this protein is associated with cancer proliferation and survival.
Figure 9: Immunofluorescence of HeLa cells using Anti-Cytokeratin 8 Antibody [K8/383] (A249150) in green. Nuclei are stained with RedDot in red.
Figure 10: IHC of human endometrial carcinoma with Anti-MUC1 Antibody [MUC1/955] (A249396).
Because epithelial cells are typically part of larger tissue structures, they are most commonly studied by Immunohistochemistry (IHC). IHC allows the rapid identification of specific epithelial sub-types and measuring the change in expression of surface markers important for disease diagnosis and prognosis. Prepared patient tissue in the form of whole tissue or biopsies allows the visualization of tissue structure and cell make-up, with fluorescent IHC adding useful details such as the location of markers on and within individual cells. This is particularly relevant to epithelial cells due to their distinct polarities, which affects the types of proteins present across the cell.
Once epithelial cells have been detached from both the extracellular/ basal matrix and each other, they can be stained with antibodies in preparation for Flow cytometry. Depending on the available equipment, 10s of markers can be identified; extracellular, intracellular and intranuclear allowing the study of the transport and expression of proteins and the isolation of specific subtypes of epithelial cell.
Diagrams created using BioRender.

![Anti-Claudin 3 Antibody [ABT-CLD3] (A97074) - IHC](https://cdn.antibodies.com/image/catalog/97/A97074_5.jpg?profile=product_image)
![Anti-Cytokeratin 19 Antibody [A53-B/A2] (FITC) (A86359) - Flow cytometry](https://cdn.antibodies.com/image/catalog/86/A86359_597.jpg?profile=product_image)
![Anti-N Cadherin Antibody [MNCD2] (A248060) - IHC](https://cdn.antibodies.com/image/catalog/248/A248060_1.jpg?profile=product_image)

![Anti-EpCAM Antibody [EGP40/837] (A249262) - Immunofluorescence](https://cdn.antibodies.com/image/catalog/249/A249262_6.jpg?profile=product_image)
![Anti-Claudin 4 Antibody [ABT-CLD4] (A97073) - IHC](https://cdn.antibodies.com/image/catalog/97/A97073_1.jpg?profile=product_image)
![Anti-Cytokeratin 8 Antibody [K8/383] (A249150) - Immunofluorescence](https://cdn.antibodies.com/image/catalog/249/A249150_3.jpg?profile=product_image)
![Anti-MUC1 Antibody [MUC1/955] (A249396) - IHC](https://cdn.antibodies.com/image/catalog/249/A249396_3.jpg?profile=product_image)