How to Choose a Secondary Antibody
Ryan Hamnett, PhD | 21st November 2024
Secondary antibodies are a type of immunoglobulin used in many research applications to bind to primary antibodies, which in turn recognize molecules of interest. Typically conjugated to fluorophores or enzyme reporters, secondary antibodies allow for highly sensitive detection of targets thanks to their specificity against the species and isotype of the primary antibody.
Selecting the right secondary antibody is critical for a successful experiment involving indirect detection of a target antigen. This guide covers the essential antibody attributes and characteristics that need to be taken into account when choosing a secondary antibody.
Jump straight to our checklist for a quick reference guide to choosing the best secondary antibody for you.
Table of Contents
Why Use a Secondary Antibody?
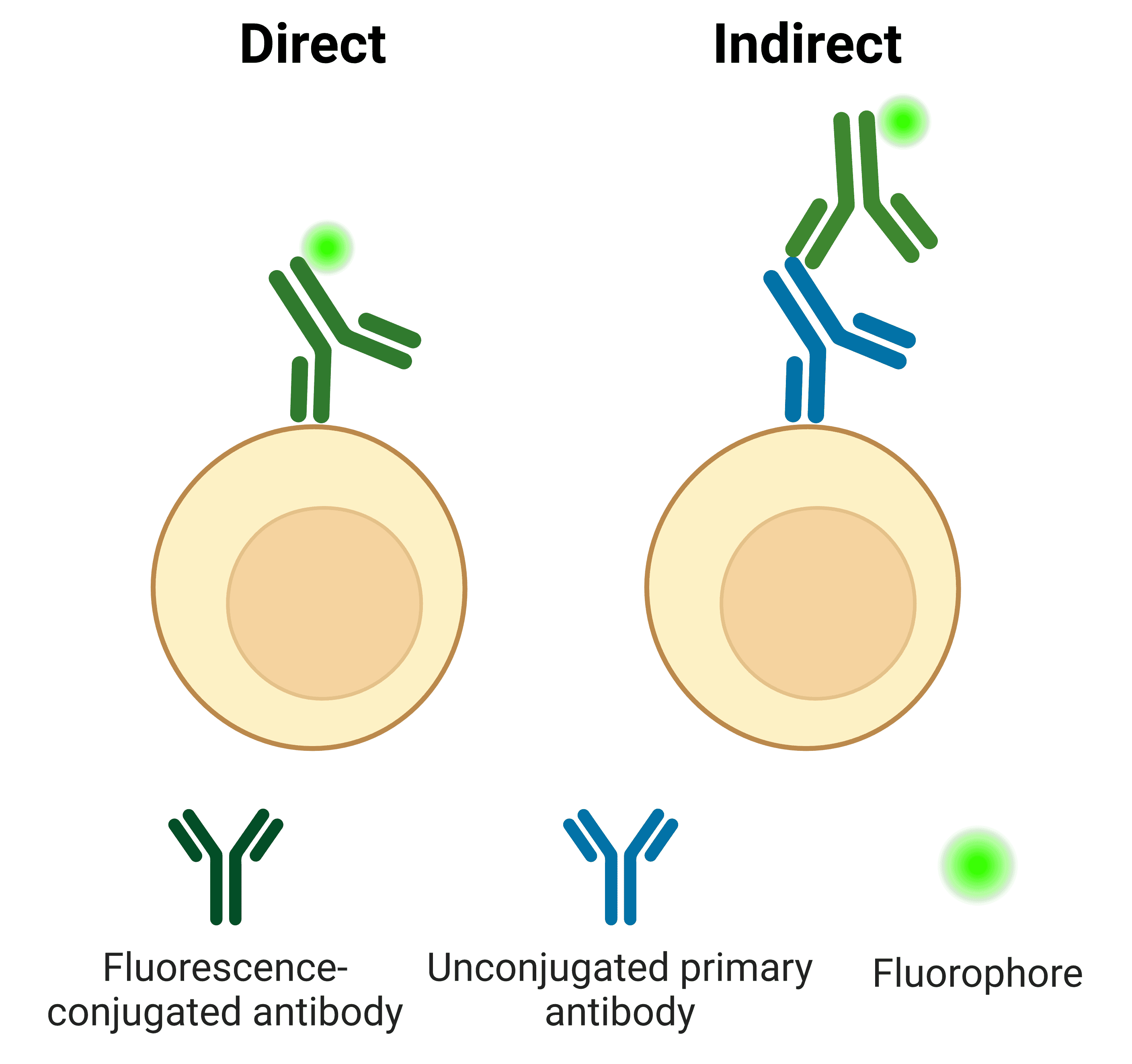
The binding properties of antibodies facilitate the detection and visualization of target molecules in applications such as immunohistochemistry and western blot. This detection can be either direct or indirect (Figure 1). For direct detection, a primary antibody raised against the target is conjugated to a fluorophore or enzyme. For indirect detection, an unconjugated primary antibody is bound by a conjugated secondary antibody.
Figure 1: Antigens can be detected directly by conjugated primary antibodies, or indirectly using secondary antibodies.
Indirect detection using secondary antibodies has several advantages over direct detection methods, including:
- Signal amplification: Multiple secondary antibodies can bind to a single primary antibody, resulting in more fluorophores per antigen
- Flexibility: A single secondary antibody can be paired with many different primary antibodies (from the same host), removing the need for each of those primary antibodies to be conjugated
- Expanded conjugate options: Because of their flexibility and general use, obtaining a secondary antibody with a less popular or unusual conjugate is much easier than obtaining a primary antibody with that conjugate
- Expands primary antibody uses: The same primary antibody can be paired with different secondary antibodies, suited to different applications (e.g. with an HRP-conjugate for western blot, or a fluorophore for immunofluorescence)
The main disadvantage of indirect detection is the potential for increased background or non-specific signal due to off-target binding of the secondary antibody.
1. Determine the host species of the primary antibody
A secondary antibody has been raised to be specific for antibodies derived from a particular host species. This is its reactivity. For example, a primary antibody raised in rabbit can be detected by an anti-rabbit secondary antibody.
2. Select the secondary antibody host species
Most secondary antibodies are raised in goat or donkey, though some other species are available too. For most applications there is little difference in the quality or binding affinity of antibodies from different hosts. Hosts should, however, be compatible with each other. In multiplexed experiments it is generally recommended to select secondary antibodies from the same host to reduce cross-reactivity (although cross-adsorbed antibodies can also be used).
3. Determine the isotype and format of the primary antibody
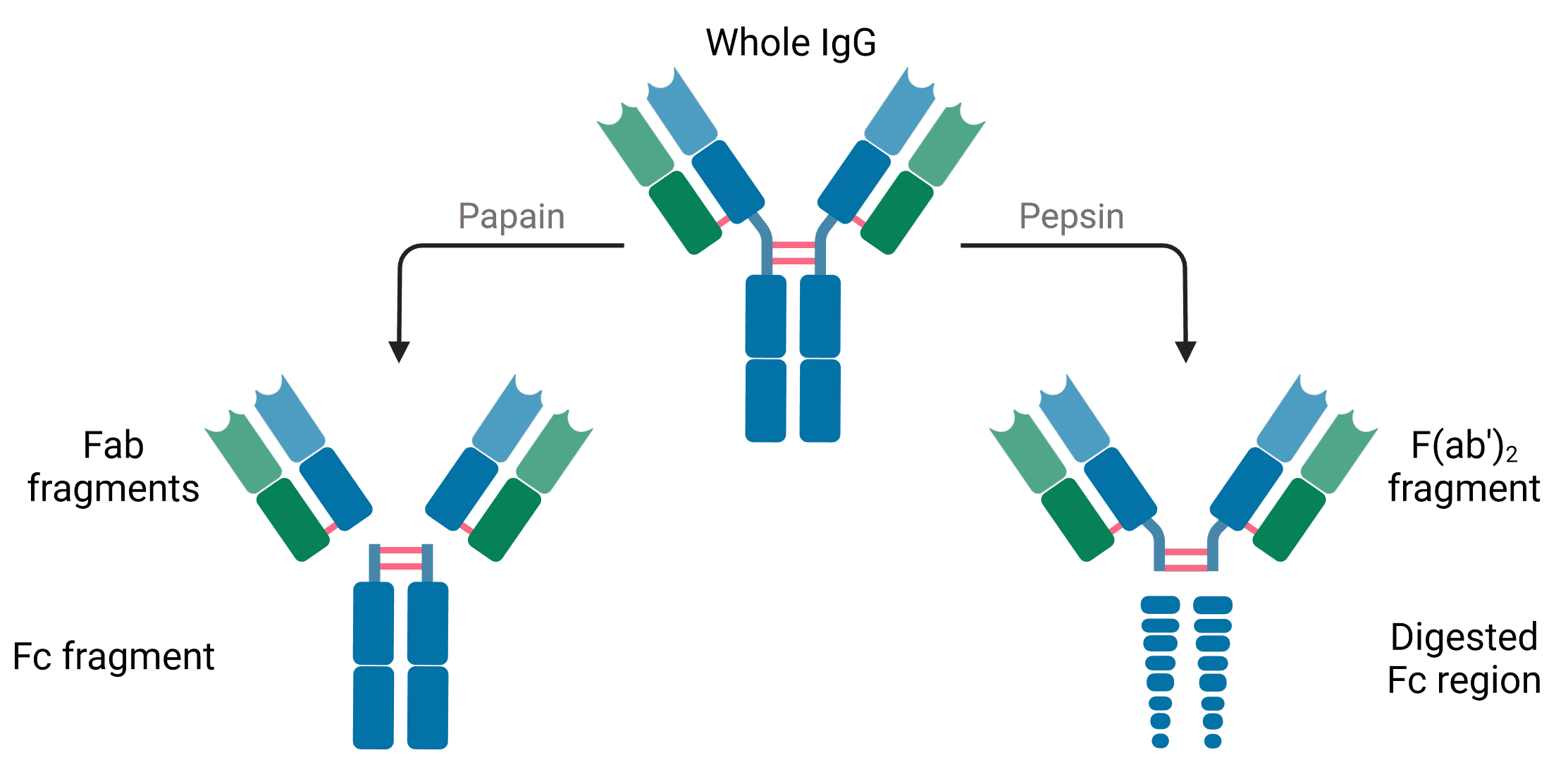
Like recognizing the primary antibody’s host species, secondary antibodies are also specific to the isotype and format of the primary antibody. For more information on isotypes and formats, see Antibody Structure, Isotypes and Formats.
There are five main antibody isotypes in mammals, determined by the antibody structure and the identity of its heavy chain: IgA, IgD, IgE, IgG and IgM. Secondary antibody specificity should match the isotype of the primary antibody. Most commercially available antibodies are IgG, though there are further subclasses such as IgG1 and IgG2 that a secondary antibody may be specific for.
Secondary antibodies can also be raised against specific structural elements of the primary antibody. These include the heavy chain, light chain, both chains (H+L), or specific regions or formats such as Fab fragments.
"Anti-IgG (H+L)" antibodies react with the heavy and light chain of IgGs, and are recommended for the majority of immunoassays. The reasons for using an isotype- or format-specific secondary antibody include:
- Reducing off-target binding: For example, an Fcγ-subclass specific antibody will exhibit minimal cross-reactivity with other subclasses.
- Multiplexing: Can be achieved using secondary antibodies specific to different isotypes rather than hosts. This is particularly useful if the primary antibodies are monoclonal or recombinant, which are will often be of a specific subclass.
- Application-specific reasons: Such as using light-chain specific secondary antibodies following immunoprecipitation to avoid interference from heavy chains.
- An antibody is the main antigen
5. Select an Appropriate Conjugate
Secondary antibodies can be conjugated to different types of molecule depending on the application they will be used with and the detection method used in that application.
Fluorophores are directly visualized, such as with a fluorescence microscope for immunocytochemistry/immunofluorescence (ICC/IF). In contrast, enzyme reporters such as horseradish peroxidase (HRP) and alkaline phosphatase (AP) generate either a colored (chromogenic) signal, such as in ELISA or immunohistochemistry (IHC), or light (chemiluminescence), such as in western blot, depending on the substrate.
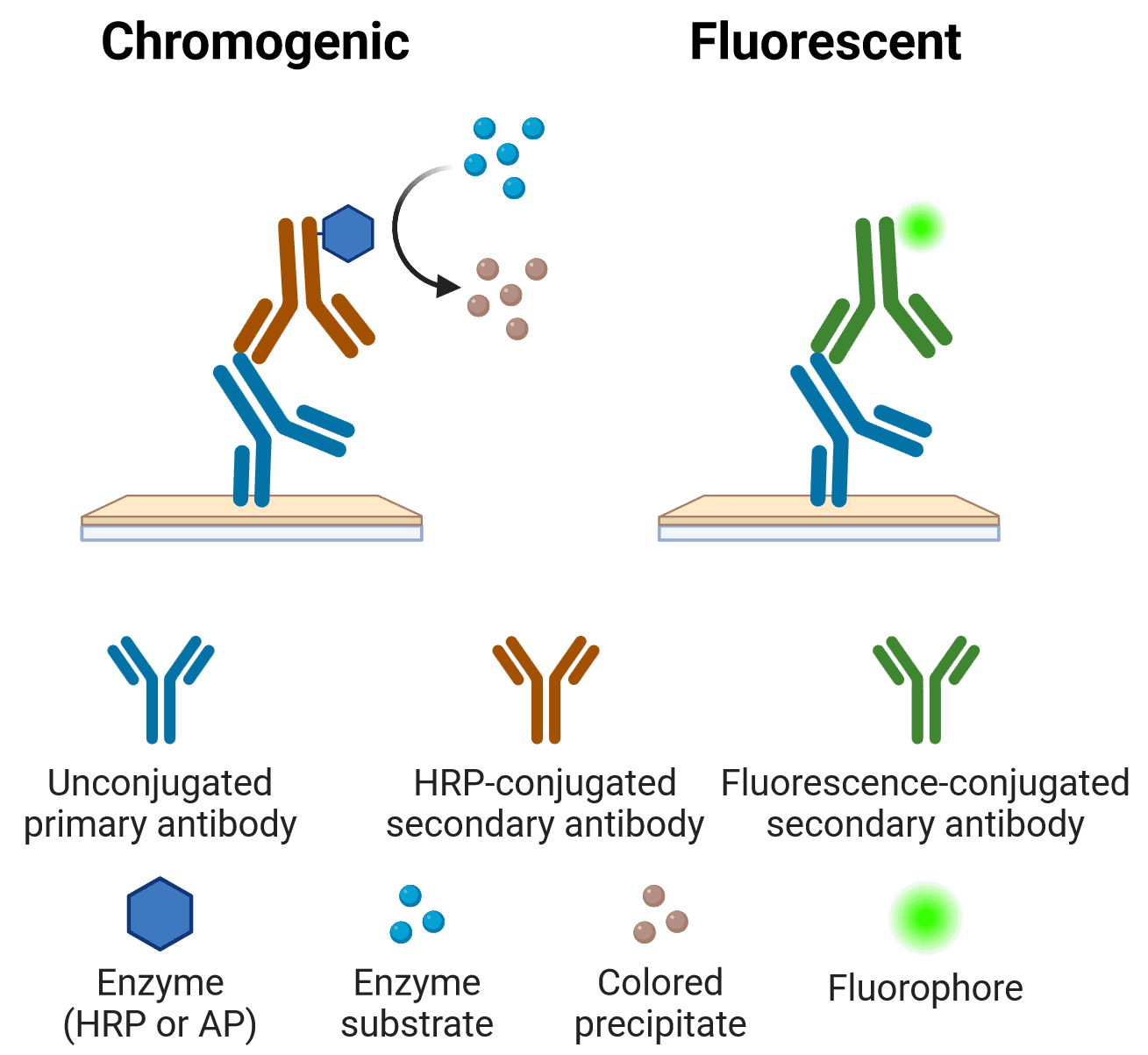
Note that most applications are compatible with more than one type of conjugate. For example, Figure 3 depicts the main detection methods for IHC.
Figure 3: Chromogenic and fluorescent indirect detection methods in IHC
The choice of conjugate will depend on the requirements of the experiment (e.g. sensitivity, resolution, detection equipment). These factors include:
- Imaging or detection equipment available: This will determine whether fluorescence, chromogenic or chemiluminescence is appropriate, in addition to other factors below
- Application: Some reporters are more commonly used in certain techniques. See Table 3 below.
- Physical characteristics of the conjugate: For example photostability and brightness for fluorophores
- Amplification requirements: Biotin-avidin systems can be used for low abundance targets for enhanced signal amplification
- Multiplexing: Colors chosen must be distinguishable from each other to reduce spectral overlap or bleedthrough
- Availability: The desired conjugate may not be available commercially. In this instance, performing your own conjugation using unconjugated secondary antibodies is an option
Fluorescent Conjugates
Fluorescent conjugates are ideal for experiments in which spatial resolution is important, such as IHC and ICC/IF, allowing the precise localization of target antigens. Table 1 shows fluorophores offered by Antibodies.com as secondary antibody conjugates.
| Fluorophore | Maximum Excitation (nm) | Maximal Emission (nm) |
| iFluor 488 | 491 | 516 |
| FITC | 490 | 525 |
| Cyanine 3 | 554 | 566 |
| TRITC | 557 | 576 |
| PE | 490; 565 | 578 |
| APC | 650 | 661 |
| Cyanine 5 | 647 | 665 |
| Cyanine 5.5 | 675 | 694 |
Table 1: Fluorescent conjugates
Enzymatic Conjugates
HRP and AP both convert a substrate into either a colored product or light for detection in a wide range of applications including ELISA, western blot and IHC.
| Enzyme | Substrate Type | Substrates | Applications |
| Horseradish peroxidase (HRP) | Chromogenic, precipitating | DAB, 4-CN, AEC | IHC, Western blot |
| Chromogenic, soluble | TMB, OPD, ABTS | ELISA |
| Fluorogenic | ADHP | ELISA |
| Luminescent | Luminol | Western blot |
| Alkaline phosphatase (AP) | Chromogenic, precipitating | BCIP/NBT, Fast Red | IHC, Western blot |
| Chromogenic, soluble | pNNP | ELISA |
| Fluorogenic | 4-MUP | ELISA, IHC |
Table 2: Enzymatic conjugates
Biotin and Amplification
Secondary antibodies can also be biotinylated, which means that they have multiple biotin molecules covalently bonded to them. Biotin has an extremely strong affinity for avidin and streptavidin. The end result of using biotinylated antibodies is significant signal amplification, because a single secondary antibody can be bound by multiple avidin or streptavidin, which in turn can be bound to multiple reporter enzymes or even to other avidin-biotin-enzyme complexes. Biotinylated antibodies and signal amplification protocols are common when trying to detect low abundance targets, such as in ELISA and IHC.
Multiplexing
Multiplexing refers to the detection of multiple target antigens at the same time. It is a common approach in IHC and flow cytometry in order to maximize the use of precious samples, and to determine colocalization or co-expression between different targets.
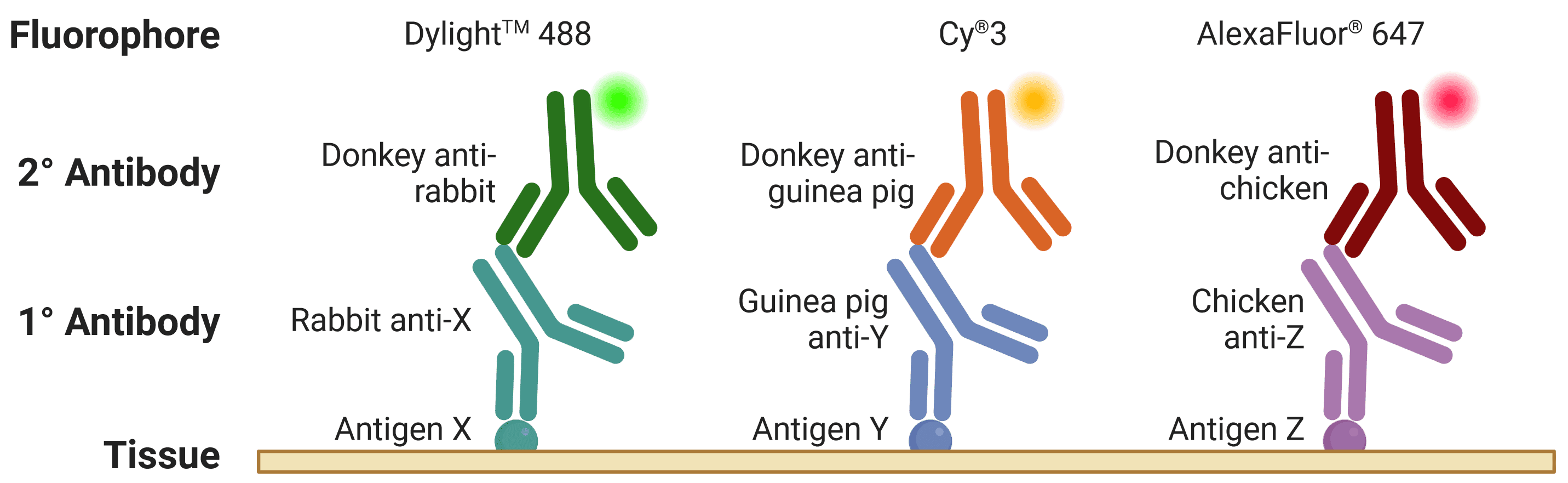
The host species of the primary antibody is extremely important to take into account when multiplexing, because secondary antibodies are directed against the host species of primary antibodies (Figure 4). Therefore, different primary antibodies should be raised in different host species when performing a multiplexed experiment to avoid cross-reactivity. For example, if two primary antibodies from the same host species are used, secondary antibodies will recognize both primary antibodies, making them impossible to distinguish during visualization. This is further complicated by secondary antibodies recognizing other secondary antibodies (e.g. donkey anti-goat secondary antibodies will bind both primary and secondary antibodies raised in goat).
![Diagram of multiplexed IHC, showing fluorophore-conjugated secondary antibodies recognizing primary antibodies from different host species]()
Figure 4: An ideal multiplexed experiment in IHC. The three primary antibodies are raised in different hosts, while the three secondary antibodies are raised in the same host and will be specific only for their target species. The three antigens can be resolved under a fluorescence microscope thanks to spectrally distinct fluorophores.
Conjugates by Application
| Application | Fluorescence | Enzyme | Biotin |
| IHC | Yes | HRP and AP for chromogenic | Yes, e.g. ABC amplification |
| ICC/IF | Yes | HRP for tyramide signal amplification | |
| ELISA | Rarely – usually only for ELISA multiplex arrays | Chromogenic or, less commonly, chemiluminescent | Yes, very common |
| Western blot | Yes, particularly useful for quantification and multiplexing | Most commonly HRP for chemiluminescence | |
| Flow cytometry | Yes | | |
Table 3: Commonly used conjugates by application
6. Determine if Cross-Adsorption is Necessary
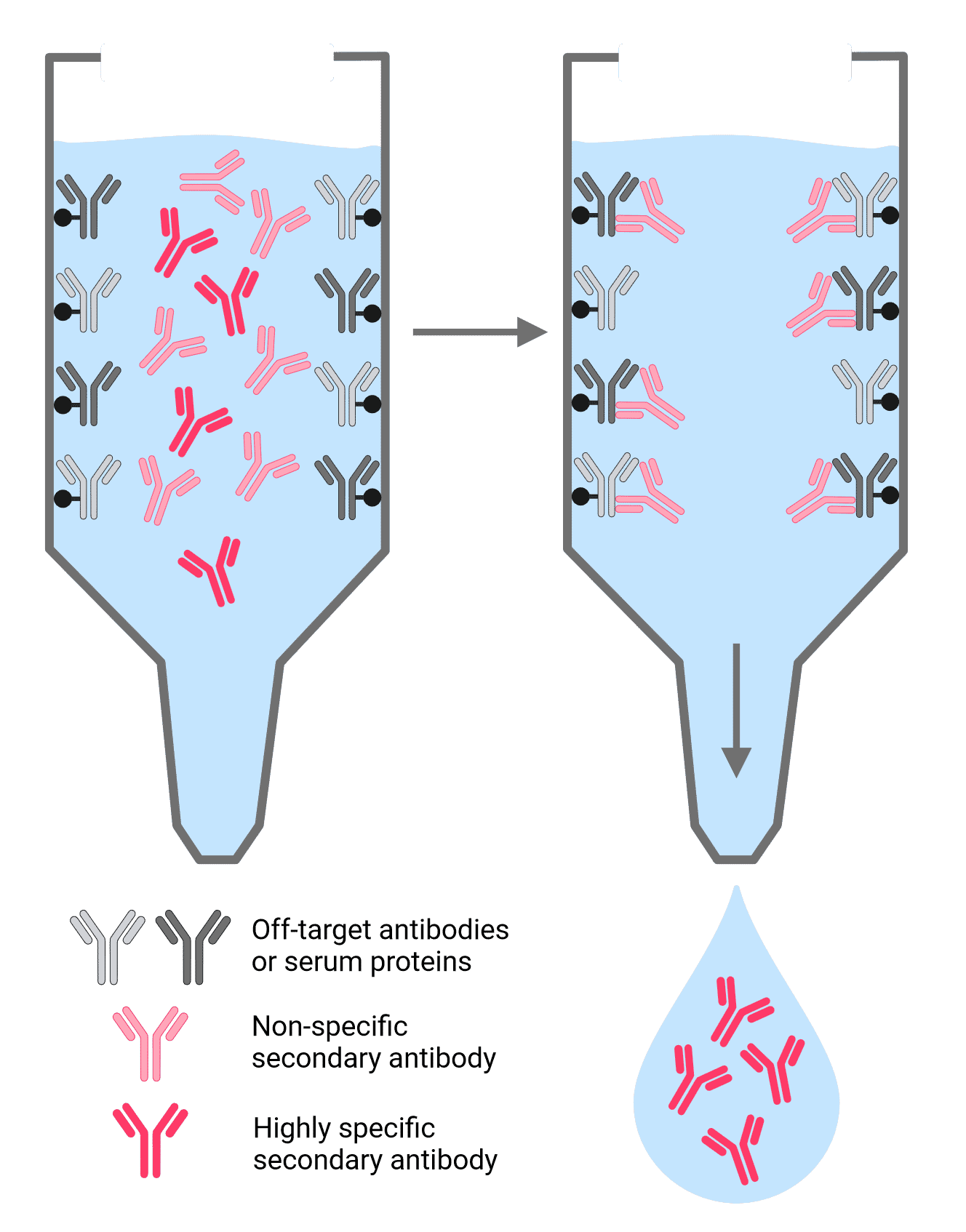
Cross-reactivity occurs when a secondary antibody recognizes off-target proteins or primary antibodies. Cross-adsorbed secondary antibodies (or pre-adsorbed antibodies) exhibit reduced cross-reactivity because they have undergone a purification process that filters out antibodies that bind to off-target serum proteins and immunoglobulins (Figure 5).
Figure 5: Antibody cross-adsorption process. Antibodies are passed through a column matrix containing immobilized proteins that are potentially cross reactive. These can include serum proteins from other species and off-target immunoglobin isotypes that share conserved sequences or structure with the target antibody. Non-specific antibodies remain bound to the column, while highly specific cross-adsorbed secondary antibodies flow through.
Any scenario in which proteins from multiple closely related species are present may benefit from cross-adsorption. These include targeting a specific antibody isotype or format, and multiplexed experiments. For example, when detecting a mouse primary antibody while multiplexing with a rat primary antibody, an anti-mouse secondary antibody may also recognize the rat primary if it has not been cross-adsorbed against rat immunoglobulins.
Cross-adsorbed secondaries have slightly reduced epitope recognition, so they should only be used when necessary.
Secondary Antibody Checklist
- Determine the host species of the primary antibody
- Select the secondary antibody host species – usually either goat or donkey.
- Determine isotype and format of the primary antibody.
- Determine secondary antibody format – whole IgG, Fab or F(ab’)2 fragments.
- Select an appropriate conjugate – Fluorescent, enzymatic or biotin. The molecule that a secondary antibody is conjugated to for detection depends on several factors, including the application, detection equipment, multiplex design, and if amplification is needed.
- Consider cross-adsorption requirements – Cross-adsorbed antibodies are useful if cross-reactivity (with tissue or with other antibodies) is likely.
Secondary Antibody Nomenclature
A lot of information is contained within the names of secondary antibodies. Consider Goat Anti-Rabbit IgG H&L Antibody (HRP), Cross-Absorbed as an example:
- Goat: Host species of the secondary antibody
- Anti-Rabbit IgG: The species of the primary antibody that the secondary antibody is directed against, and the isotype it is directed against (IgG)
- H&L: Indicates specificity for both the heavy and light chains of the primary antibody
- (HRP): Describes the conjugate, in this case the horseradish peroxidase enzyme
- Cross-Adsorbed: Antibody has been adsorbed to the serum of many different species to reduce cross-reactivity, details of which are on the datasheet.
Frequently Asked Questions (FAQ)
There are no differences in antibody structure between primary and secondary antibodies. The only difference is in how they are used. Primary antibodies bind to a target antigen, while secondary antibodies bind to primary antibodies.
The recommended dilution for a secondary antibody is usually displayed in the product datasheet, and will vary with application. The values below can be used as starting points:
- Western Blot 1:5000 - 1:50000
- ELISA 1:1000 - 1:1000000
- ICC/IF/IHC 1:100 - 1:1000
This is a control most commonly encountered in IHC and ICC/IF. Because secondary antibodies have the potential to bind to non-specific targets, it is important to determine the extent to which such off-target binding is contributing to the observed signal. This can be done by omitting the primary antibody but still including the secondary antibody. Any signal seen in this control sample has been generated by non-specific secondary antibody binding.
This article is part of our comprehensive Antibody Basics guide: