Antibody conjugation kits chemically attach reporter molecules, such as enzymes or fluorescent dyes, to primary antibodies. Conjugated primary antibodies are used in direct immunoassays to visualize or detect protein targets directly, without the need for secondary antibodies.
Antibody conjugation kits are most effective when working with highly pure antibodies that lack additives such as BSA and azide in their formulation. Antibodies.com offers over 8,000 carrier-free antibodies that are ready for conjugation.
Our StreptaClick antibody conjugation kits use click chemistry and a novel monomeric streptavidin to quickly generate highly pure and sensitive conjugated primary antibodies. StreptaClick kits are not restricted to specific antibody host species or isotypes and can be used with IHC, ICC/IF and flow cytometry, providing a fast and flexible procedure for labeling antibodies with fluorophores or horseradish peroxidase (HRP).
Common antibody labels include fluorescent dyes, enzymes and biotin, with the exact choice often determined by the application, detection method, and experimental aims.
| Application | Conjugate(s) |
|---|---|
| Immunohistochemistry | Fluorescent dyes, HRP, AP, biotin |
| Immunocytochemistry/Immunofluorescence | Fluorescent dyes |
| Flow cytometry | Fluorescent dyes, fluorescence proteins (e.g. PE, APC), tandem dyes |
| ELISA | HRP, biotin |
| Western blot | HRP, AP, fluorescent dyes |
Fluorescent conjugates produce fluorescent signals of specific wavelengths, making multiple, spectrally distinct fluorophores an ideal option for multiplexing in IHC, ICC/IF and flow cytometry. Antibodies.com offers a wide variety of fluorophore conjugation kits that use NHS ester, sulfhydryl (SH) or click chemistry to label primary antibodies.
Note that AZDyes 488, 594 and 647 are structurally identical to Alexa Fluor 488, Alexa Fluor 594 and Alexa Fluor 647, respectively.
When conjugated to antibodies, enzymes such as horseradish peroxidase (HRP) and alkaline phosphatase (AP) enable detection and visualization by catalyzing reactions that produce chromogenic, fluorescent or chemiluminescent products, depending on the application and substrate.
The most commonly used fluorescent substrate for HRP are tyramide signal amplification (TSA) reagents. HRP catalyzes the conversion of inactive, labeled tyramide to a reactive radical, which then binds nearby tyrosine residues at the site of the antibody-antigen complex. These reagents are included in the kits indicated below, or can be purchased separately such as in our Tyramide Signal Amplification Kit - 3 Color (A329042) and Tyramide Signal Amplification Kit - 5 Color (A329043) kits.
| Product | Conjugate | Applications | Catalog No. |
|---|---|---|---|
| Peroxidase Labeling Kit - NH2 | HRP | ELISA, western blot, IHC | A57319 |
| Peroxidase Labeling Kit - NH2 (for 1mg) | HRP | ELISA, western blot, IHC | A57308 |
| Peroxidase Labeling Kit - SH | HRP | ELISA, western blot, IHC | A57321 |
| Peroxidase Labeling Kit - SH (for 1mg) | HRP | ELISA, western blot, IHC | A57307 |
| StreptaClick® HRP Complete Tyramide Signal Amplification and Biotinylation Kit - 3 Color | HRP | ICC/IF, Fluorescent IHC | A329040 |
| StreptaClick® HRP Complete Tyramide Signal Amplification and Biotinylation Kit - 5 Color | HRP | ICC/IF, Fluorescent IHC | A329041 |
| Alkaline Phosphatase Labeling Kit - NH2 | AP | ELISA, western blot, IHC | A57318 |
| Alkaline Phosphatase Labeling Kit - SH | AP | ELISA, western blot, IHC | A57317 |
| Alkaline Phosphatase Labeling Kit - SH (for 1mg) | AP | ELISA, western blot, IHC | A57303 |
Note that StreptaClick® reagents rely on first biotinylating the antibody prior to conjugating HRP via a click reaction using a processed form of streptavidin.
Biotin is a small molecule that can be easily conjugated to antibodies. Once biotinylated, antibodies can make use of the extremely high affinity between biotin and avidin/streptavidin proteins to access signal amplification techniques such as the avidin-biotin complex (ABC) method. Amplification is achieved because each avidin molecule can be bound to a reporter such as HRP, multiple avidin-HRP molecules can bind to the antibody, and clusters of avidin-HRP arise through binding to each other. We offer four biotinylation kits:
Determining the fluorophore:protein ratio relies on calculating the molar concentrations of both the fluorophore and the protein (antibody). The most common method of determining the molar ratio is to use their absorbance at specific wavelengths in a spectrophotometer. The ratio between the molar concentrations of fluorophore and protein is a useful guide of the average number of fluorophore molecules conjugated to the antibody.
To calculate the fluorophore:protein ratio:
Horseradish peroxidase (HRP) and alkaline phosphatase (AP) are both compatible with western blot, ELISA, and immunohistochemistry, but each enzyme has distinct kinetics, substrates, ideal conditions and sources of interference that should be taken into account when deciding which to use.
While both HRP and AP are widely used, HRP tends to be preferred in most instances because it is cheaper, more versatile, and more established in many biochemical techniques. The table below summarizes the key features, advantages and disadvantages of HRP and AP. For a comprehensive comparison of HRP and AP substrates in IHC, see our Immunohistochemistry application guide.
| HRP | AP | |
|---|---|---|
| Chromogenic substrates | TMB (ELISA), DAB (IHC) | pNPP (ELISA), BCIP/NBT (IHC) |
| Fluorescent substrates | TSA (IHC) | Uncommon |
| Chemiluminescent substrates | Luminol-based reagents (western blot, ELISA) | Acridan- and 1,2-dioxetane-based reagents (western blot, ELISA) |
| Advantages |
|
|
| Kinetics | Very fast | Slower, linear |
| Assay time | As little as 5 minutes for signal to develop | Signal develops over 30-60 minutes for most applications |
| Size | ~40 kDA | ~140 kDA (calf intestinal AP) |
| Toxicity of substrates | Some substrates, such as OPD and DAB, are suspected mutagens and carcinogens | pNPP contains diethanolamine, which is toxic. |
| Sources of inhibition | Microorganisms and antibiotics can lead to HRP degradation. Azides (e.g. sodium azide), sulfides and cyanides can also inhibit HRP activity. | Not compatible with phosphate buffers. Inhibited by cyanide, cysteine, inorganic phosphate, arsenate and divalent chelating agents like EDTA. |
| Sources of background signal | Endogenous peroxidases, which can be quenched by hydrogen peroxide | Endogenous phosphatases, which can be quenched by levamisole |
| pH range | Functions best at neutral pH | Functions best at alkaline pH (~pH 9) |
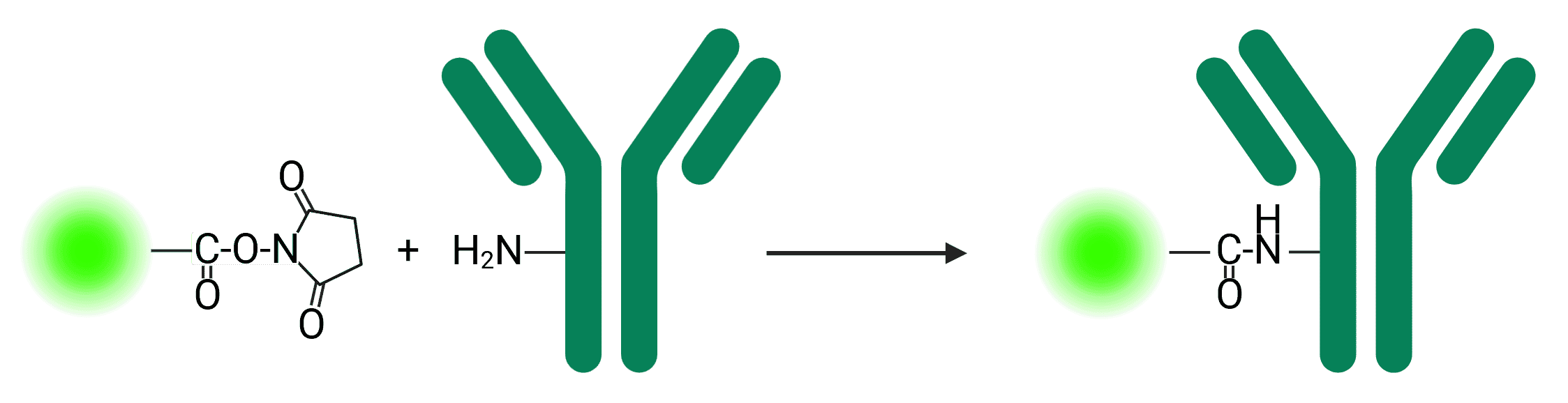
Reporter molecules can often be found with the reactive N-hydroxysuccinimide (NHS) ester group, which are widely used due to their reactivity with primary amines commonly available on proteins (see Figure 1).
Figure 1: NHS ester reaction with a primary amine to conjugate a fluorophore to an antibody
NHS ester crosslinking reactions are commonly performed in phosphate, carbonate/bicarbonate, HEPES or borate buffers at pH 7.2-8.5 for 0.5-4 h at room temperature or 4°C. Buffers that contain primary amines such as Tris buffers can compete with the reaction, but may be added as a quencher to stop the reaction following conjugation.
Chemical groups that form bonds with sulfhydryl (-SH) groups are commonly used for antibody and protein conjugation. Sulfhydryl groups, also known as thiols, are found as side chains on cysteine residues in proteins.
Compounds such as maleimides, haloacetyls and aziridines can all react with sulfhydryl groups. Only free thiols can be conjugated; thiols that are involved in disulfide bonds cannot be conjugated. Sulfhydryl groups are common on proteins but less common than primary amines, which means that conjugation to sulfhydryls is more precise. Beyond existing thiol groups, thiols can be generated through reduction of existing disulfide bonds, or introduction of thiols (such as by using Traut’s reagent).