Kai Boon Tan, PhD | 4th July 2025
Dopaminergic neurons are specialized neuronal populations that synthesize and release the neurotransmitter dopamine. They play critical roles in motor control,1,2 reward processing,3 cognition,4,5 and neuroendocrine regulation.6,7 Dysfunctions in dopaminergic neurons have been implicated in Parkinson’s disease,8–10 addiction,11 depression,12 schizophrenia,13 attention deficit hyperactivity disorder (ADHD),14,15 and hyperprolactinemia.16,17 Dopaminergic neurons can be found across distinct regions within the central nervous system and classified into nine clusters termed A8–A16.
Dopaminergic neuron markers are proteins that can be used to identify and characterize dopamine-expressing neurons in a mixed population. Below are examples of how dopaminergic neuron markers can be used in basic and translational neuroscience research:
| Molecular markers | Abbreviation | Protein Type | Description |
|---|---|---|---|
| Tyrosine Hydroxylase | TH | Enzyme | Enzyme in dopamine synthesis. The gold standard for identifying mature dopaminergic neurons across all regions. |
| Dopa Decarboxylase / Aromatic L-Amino Acid Decarboxylase | DDC / AADC | Enzyme | Enzyme in dopamine synthesis. Co-express with TH in mature dopaminergic neurons. |
| Dopamine Transporter | DAT / SLC6A3 | Transporter | Reuptakes dopamine from the synaptic cleft. Highly specific to dopaminergic terminals and soma. |
| Vesicular Monoamine Transporter 2 | VMAT2 / SLC18A2 | Transporter | Packages dopamine into synaptic vesicles. |
| Aldehyde dehydrogenase 1 family member A1 | ALDH1A1 | Enzyme | Highly enriched in human and primate substantia nigra pars compacta dopaminergic neurons compared to VTA. Less prominent in rodents. |
| Nuclear receptor related 1 protein | NURR1 / NR4A2 | Transcription factor | Critical transcription factor for the development, maturation, and maintenance of dopaminergic neurons. |
| Pituitary homeobox | PITX3 | Transcription factor | Essential for the development and survival of substantia nigra pars compacta dopaminergic neurons. |
| LIM homeobox transcription factor 1 alpha and beta | LMX1A/B | Transcription factor | Key developmental regulator of midbrain dopaminergic neuron specification. |
| Forkhead box protein A2 | FOXA2 | Transcription factor | Key developmental regulator of midbrain dopaminergic neurons. |
Table 1: Summary of common molecular markers used to characterize mature and developing dopaminergic neurons.
Dopamine neurons in the brain are primarily distributed across the midbrain and hypothalamus; the pathways of dopaminergic neurotransmission are illustrated in Figure 1.
Figure 1: Dopaminergic neurotransmission in the brain. A8-A10 project from the midbrain to the striatum (nigrostriatal pathway), limbic (mesolimbic pathway) regions and cortex (mesocortical pathway), while A11-A15 signal to other cells within the hypothalamus as well as to cells of the pituitary gland (tuberoinfundibular pathway) and spinal cord. A16 is found in the olfactory bulb and projects locally. Hyp: hypothalamus; NAc: nucleus accumbens; OF: olfactory bulb; RRF: retrorubral field; SNc: substantia nigra; VTA: ventral tegmental area.
The midbrain dopaminergic systems comprise the most prominent clusters and include the retrorubral field (RRF) (A8), the substantia nigra pars compacta (SNc) (A9), and the ventral tegmental area (VTA) (A10). The RRF dopaminergic neurons (A8) innervate the ventral striatum and amygdala, contributing to the mesolimbic and mesocortical pathways to modulate reward, motor control, fear, and pain modulation.18,19 The SNc dopaminergic neurons (A9) form the nigrostriatal pathway, projecting to the dorsal striatum to regulate voluntary movement.19,20 Degeneration of this pathway underlies Parkinson's motor symptoms.8–10 The ventral tegmental area (VTA) (A10) dopaminergic neurons project via the mesolimbic and mesocortical pathways to the nucleus accumbens and prefrontal cortex to modulate reward, motivation, and decision-making.19,21
The diencephalon or hypothalamic dopaminergic neuron clusters A11–A15 regulate neuroendocrine, autonomic, and behavioral functions. The posterior hypothalamic dopaminergic group (A11) projects to the spinal cord and modulates spinal autonomic outflow.22 The arcuate nucleus (A12) and periventricular nucleus (A14) neurons innervate the median eminence at the base of the hypothalamus, directly above the pituitary gland, to jointly inhibit prolactin secretion.23,24 The zona incerta (A13) and anteroventral periventricular nucleus (A15) dopaminergic neurons regulate nociception25 and reproductive hormone regulation,26 respectively. Additionally, olfactory bulb dopaminergic neurons (A16) act locally within the olfactory circuit and contribute to olfaction processes such as odor discrimination, olfactory learning, and memory.27,28
Table 2 summarizes key markers found in each cluster of dopaminergic neurons in the mammalian brain.
| Dopaminergic Neuron Group | Region | Primary Target | Key Markers |
|---|---|---|---|
| Retrorubral field (RRF) / A8 | Midbrain | Striatum, amygdala | TH+, DAT+, VMAT2+, DDC+, NURR1+, FOXA2+ (low), LMX1A/B+ |
| Substantia nigra pars compacta (SNc) / A9 | Midbrain | Dorsal striatum | TH+, DAT+ (High), VMAT2+, DDC+, PITX3+, ALDH1A1+ (primate-specific), NURR1+, FOXA2+, LMX1A/B+ |
| Ventral tegmental area (VTA) / A10 | Midbrain | Ventral striatum, prefrontal cortex, limbic system | TH+, DAT+ (Moderate), VMAT2+, DDC+, PITX3+, ALDH1A1+ (primate-specific), NURR1+, FOXA2+, LMX1A/B+ |
| Hypothalamic / A11-A15 | Hypothalamus | Spinal cord, pituitary, hypothalamus | TH+, DDC+, DAT- , VMAT2+ (moderate) |
| Olfactory bulb / A16 | Olfactory bulb | Periglomerular cells | TH+, DDC+, DAT-, VMAT2+ (moderate) |
| Retinal | Retina | Amacrine cells | TH+, DAT-, VMAT2- |
Table 2: Expression of dopaminergic neuron markers in various brain regions
TH is the rate-limiting enzyme in dopamine synthesis (Figure 2). It catalyzes the conversion of L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA), the immediate precursor of dopamine.29 As the first and most critical step in the production of monoamine neurotransmitters, TH is exclusively expressed in dopaminergic,30 noradrenergic,31 and adrenergic neurons.32,33 For dopaminergic neurons, TH serves as the gold-standard pan-dopaminergic marker across the different brain regions, ranging from the midbrain to hypothalamic and olfactory bulb dopaminergic neuron populations.
Figure 2: Dopamine synthesis and metabolism in neurons. Dopamine (DA) is synthesized within dopaminergic neurons from tyrosine via L-DOPA by the action of tyrosine hydroxylase (TH) and aromatic amino acid decarboxylase/dopa decarboxylase (AADC / DDC). DA is loaded into vesicles by VMAT2 for synaptic release, with excess DA taken up by neurons for re-use through the dopamine transporter (DAT), or by astrocytes. In neurons or glia, dopamine can be degraded by catechol-o-methyltransferase (COMT) or monoamine oxidase (MAO) to form homovanillic acid (HVA) or be oxidized to form DOPAL and DOPAC metabolites and hydrogen peroxide (H2O2). TH, DDC, VMAT2 and DAT are all common markers of dopaminergic neurons. Reproduced under Creative Commons 4.0 CC-BY from Xu, H., Yang, F. The interplay of dopamine metabolism abnormalities and mitochondrial defects in the pathogenesis of schizophrenia. Transl Psychiatry 12, 464 (2022).
Figure 3: IHC of rat brain tissue stained with Anti-Tyrosine Hydroxylase Antibody (A104316) in green. Nuclei are marked by DAPI in blue.
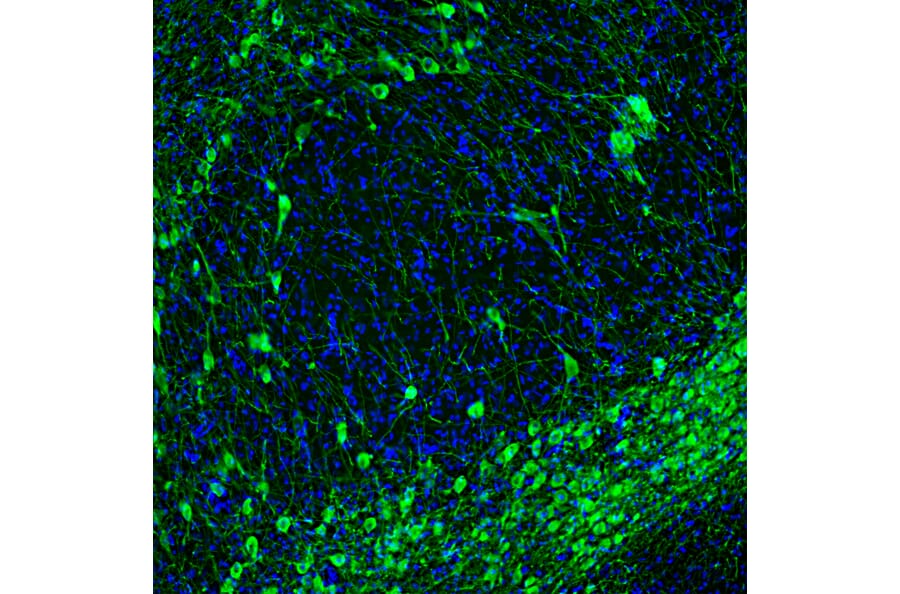
Figure 4: IHC of mouse brain tissue stained with Anti-Tyrosine Hydroxylase Antibody [4H2] (A104315).
Dopa decarboxylase (DDC), also known as aromatic L-amino acid decarboxylase (AADC), is a pyridoxal phosphate-dependent enzyme responsible for synthesizing key monoamine neurotransmitters.34 DDC is expressed in dopaminergic neurons to catalyze the decarboxylation of L-DOPA to dopamine.35
Clinically, DDC is targeted by peripheral inhibitors like carbidopa to enhance central dopamine delivery in Parkinson’s disease (PD), preventing peripheral L-DOPA degradation.
Figure 5: IHC of rat brain tissue stained with Anti-DOPA Decarboxylase/DDC Antibody (A14470).
DAT, encoded by the SLC6A3 gene, is a presynaptic membrane transporter that regulates extracellular dopamine levels via sodium-dependent reuptake into dopaminergic neurons.36 DAT terminates dopaminergic signaling by rapidly clearing synaptic dopamine, crucially modulating reward, motivation, and motor control pathways.37 DAT is highly enriched in striatal terminals of SNc (A9) dopaminergic neurons, with relatively lower expression in the VTA (A10) and cortical projections. Clinically, DAT dysfunction is implicated in Parkinson’s disease,38 ADHD,39 and addiction.40
VMAT2, encoded by SLC18A2, is a proton-dependent transporter that packages monoamine neurotransmitters, including dopamine, serotonin, norepinephrine, and histamine, into synaptic vesicles for presynaptic storage and regulated release.41,42 With this, VMAT2 maintains neurotransmitter homeostasis by preventing cytoplasmic dopamine degradation.41 VMAT2 usually localizes to vesicular membranes of dopaminergic neurons and neuroendocrine cells.
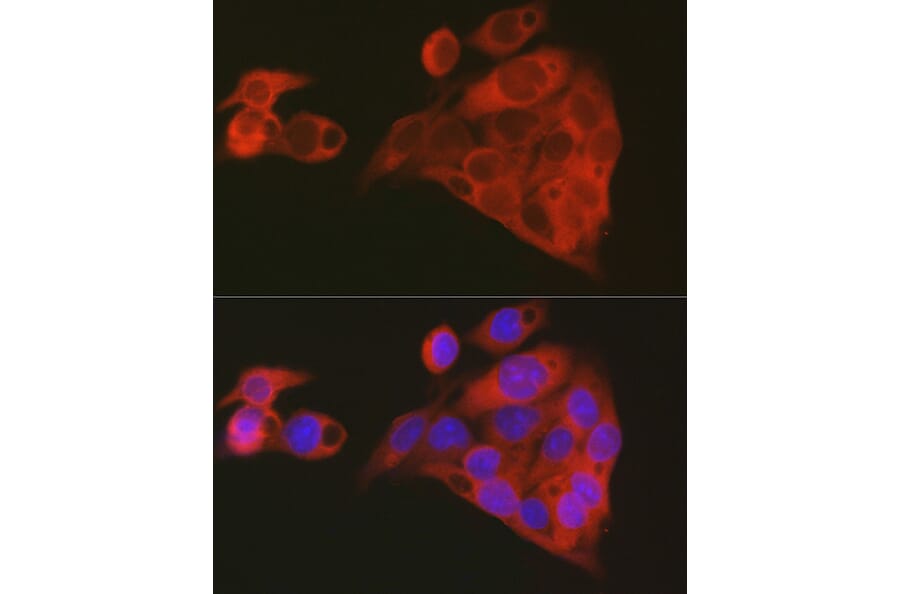
In dopamine metabolism, ALDH1A1 is a neuroprotective enzyme that catalyzes the oxidation of the highly reactive aldehyde 3,4-dihydroxyphenylacetaldehyde (DOPAL) to 3,4-dihydroxyphenylacetic acid (DOPAC), a benign metabolite that can be excreted from the dopaminergic neuron.43 Without ALDH1A1, DOPAL accumulates in the dopaminergic neurons and leads to α-synuclein aggregation, a hallmark of Parkinson’s disease.44
In translational research, ALDH1A1 is a selective marker for the vulnerable SNc (A9) dopaminergic neuron population that undergoes selective degeneration in Parkinson's disease in humans45 and the non-human primate disease model, where its expression correlates with neuromelanin synthesis and mitochondrial function.46
Figure 6: IF of HepG2 cells stained with Anti-ALDH1A1 Antibody (A13666).
NURR1, encoded by the NR4A2 gene, is an orphan nuclear receptor essential for the development, maintenance, and survival of midbrain dopaminergic neurons. It regulates the expression of key dopaminergic genes, including TH, DAT, and VMAT2, ensuring proper dopamine synthesis, reuptake, and storage.47 During development, NURR1 is critical for the terminal differentiation and maturation of SNc (V9) and VTA (V10) dopaminergic neurons.48 In adulthood, NURR1 continues to protect dopaminergic neurons by maintaining mitochondrial function and reducing oxidative stress.49 Clinically, mutations in the NR4A2 gene are linked to familial forms of Parkinson's disease,50 while pharmacological activation of NURR1 has shown neuroprotective effects in preclinical Parkinson's disease models.51
FOXA2 is a key transcription factor regulating the specification, survival, and functional maintenance of midbrain dopaminergic neurons. FOXA2 is active from early development, cooperating with LMX1A/B and OTX2 to establish midbrain dopaminergic fate in the ventral midbrain floor plate.52 FOXA2 regulates transcription of genes such as TH and DDC for dopamine synthesis, and NURR1 and PITX3 for neuronal survival.53 In adults, FOXA2 continues to protect midbrain dopaminergic neurons by mitigating oxidative stress and sustaining mitochondrial function.54 FOXA2 dysfunction is linked to Parkinson’s disease, where reduced FOXA2 expression correlates with SNc neurodegeneration.55 FOXA2 also guides stem cell-derived DA neuron differentiation, making it a key target for cell replacement therapies.56


![IHC - Anti-Tyrosine Hydroxylase Antibody [4H2] (A104315)](https://cdn.antibodies.com/image/catalog/104/A104315_3.jpg?profile=product_image)