Kai Boon Tan, PhD | 1st May 2025
Interneurons are neurons that enable communication between other neurons and play central roles in neural circuit function, mediating signal integration and reflex circuits within the nervous system. Interneurons vary widely in neurotransmitter type, function and region within the nervous system. The classification of interneurons by neurotransmitter types is often associated with their specialized roles across different regions characterized by distinct cellular properties and synaptic dynamics.
Interneurons can be defined by their expression of characteristic protein markers. This guide highlights the most widely used interneuron markers suitable for most experimental applications.
Below are examples of how interneuron markers can be used in Neuroscience research:
Inhibitory interneurons are by far the most common type in the central nervous system, especially in the cortex. Inhibitory interneurons are highly heterogeneous and play crucial roles in orchestrating neural networks in the central nervous system. In the cerebral cortex, inhibitory interneurons come with diverse morphologies, connectivity, biochemistry and physiological properties, and constitute 20-30% of the cortical neuronal population.1,2 Virtually all inhibitory interneurons are GABAergic, characterized by their synthesis and release of γ-aminobutyric acid (GABA), which mediates inhibitory control over excitatory glutamatergic neurons.
GABAergic interneuron subclasses can be identified by using combinations of antibodies targeting specific molecular markers, as summarized in Table 1. In the cerebral cortex, all GABAergic interneuron classes exclusively express either parvalbumin (PV), somatostatin (SST), or serotonin receptor 3A (5HT3A),3,4 making these markers essential in classifying GABAergic interneuron subclasses in combination with other subclass-specific markers.
For a more comprehensive overview, please refer to the Lim et al. (2018) review article,4 as well as single-cell transcriptomic studies,1,2 which provide nuanced insights into molecular markers specific to GABAergic interneuron subclasses.
| Molecular Marker | Protein Type | Description |
|---|---|---|
| Glutamate decarboxylase (GAD65, GAD67) | Enzyme | Essential for GABA biosynthesis |
| Parvalbumin (PV) | Calcium-binding protein | Labels fast-spiking interneurons (e.g., basket and chandelier cells) |
| Somatostatin (SST) | Neuropeptide | Marks SST-expressing Martinotti, non-Martinotti and long-projecting neurons |
| Serotonin receptor 3A (5HT3A) | Receptor | Marks all 5HT3A-expressing interneurons, including VIP-, CCK- and RELN-expressing interneurons |
| Calretinin (CR) | Calcium-binding protein | Marks dendrite-targeting interneurons like SST-expressing Martinotti cells and 5HT3A-expressing bipolar cells |
| Calbindin (CB) | Calcium-binding protein | Marks a subpopulation of SST-expressing Martinotti cells |
| Vasoactive intestinal peptide (VIP) | Neuropeptide | Mark 5HT3A-expressing basket and bipolar cells that targets PV and SST interneurons for disinhibition |
| Cholecystokinin (CCK) | Neuropeptide | Mark a subpopulation of 5HT3A-expressing basket cells |
Table 1: Summary of common molecular markers used to characterize GABAergic inhibitory interneurons.
Pan-GABAergic Interneuron Markers
Glutamate decarboxylase (GAD) is an enzyme responsible for synthesizing the inhibitory neurotransmitter GABA released by inhibitory interneurons.5,6 GAD67 (encoded by Gad1) and GAD65 (encoded by Gad2) serve as pan-interneuron markers to characterize inhibitory interneurons across different nervous system regions. Although both isoforms are expressed in inhibitory neurons, GAD67 is constitutively active and widely distributed in soma and dendrites,7 whereas GAD65 is expressed at synaptic terminals and dynamically regulated during neurotransmission.8 Their broad expression makes them essential tools for delineating GABAergic interneurons from excitatory glutamatergic neurons in immunohistochemistry (IHC), Western blot (WB), and flow cytometry. For a more comprehensive overview of pan-GABAergic interneuron markers, please refer to the GABAergic Neuron Markers page.
Figure 1: IHC of rat brain tissue stained with Anti-GAD65 Antibody (A12729) in red. Nuclei were stained by DAPI in blue.
Figure 2: IHC of rat eye cells stained with Anti-GAD67 Antibody (A14262) in red. Nuclei were stained by DAPI in blue.
Parvalbumin (PV)
PV is a calcium-binding protein that serves as a key molecular marker for a major interneuron class that makes up approximately 40% of interneurons in the cortex1. PV-expressing (PV+) interneurons are functionally diverse and include subclasses such as basket, chandelier, and translaminar interneurons, each targeting distinct compartments of principal neurons and playing critical roles in modulating network oscillations,9 synaptic plasticity10 and excitatory-inhibitory balance.11,12 While most PV interneurons are classically defined as fast-spiking interneurons, there are also non-fast-spiking PV interneuron subclasses such as a layer 4 PV interneuron subclass.13 IHC of PV helps to distinguish PV interneurons from other inhibitory neuron classes, locate their layer distribution and study their connectivity and co-expression with other markers.
Figure 3: IHC of rat hippocampal tissue stained with Anti-Parvalbumin Antibody [3C9] (A85317) in red and Anti-Calretinin Antibody (A104312) in green. Nuclei were stained by DAPI in blue.
Figure 4: IHC of rat eye cells stained with Anti-Parvalbumin Antibody (A85316) in green and Anti-GFAP Antibody (A85422) in red. Nuclei were stained by DAPI in blue.
Somatostatin (SST)
SST is a neuropeptide widely expressed in the nervous system and acts as both neurotransmitter and neuromodulator to regulate network activity.14 In the cortex, SST marks a major interneuron class, known as SST-expressing (SST+) interneurons or SST interneurons, that constitutes 30% of the cortical interneuron population.
Figure 5: IHC of rat brain tissue stained with Anti-Somatostatin Antibody [ARC50670] (A308885).
Figure 6: IHC of rat pancreas cells stained with Anti-Somatostatin Antibody (A16303) in orange. Nuclei were stained by DAPI in blue.
SST interneurons, which encompass the well-characterized subclasses Martinotti, non-Matinotti and long-projecting neurons, predominantly target the distal dendrites of pyramidal neurons, thereby modulating excitatory input,15 shaping sensory processing,16 and regulating cortical network activity.17,18 SST interneurons are also essential for excitation-to-inhibition balance, synaptic plasticity, learning,19 and memory.20 IHC of SST can be applied in combination with calretinin (CR) and calbindin (CB) to characterize Martinotti interneurons (SST+/CR+/CB+); and with nitric oxide synthase (NOS) to identify long-projecting SST interneurons (SST+/NOS+).
Figure 7: IHC of rat cerebellum stained with Anti-Calretinin Antibody [3G9] (A85367) in green and Anti-Calbindin Antibody (A85359) in red.
Figure 8: IHC of rat cerebellum stained with Anti-Calbindin Antibody [5A9] (A85362) in red and Anti-GFAP Antibody (A85419) in green. Nuclei were stained by DAPI in blue.
Serotonin receptor 3A (5HT3A)
5HT3A is an ionotropic serotonin receptor that directly controls ion channels for rapid synaptic transmission upon the binding of serotonin.21 5HT3A is predominantly expressed by a major interneuron class derived from a transient embryonic brain region, the caudal ganglionic eminence,22 in contrast to the PV and SST interneuron classes generated from the medial ganglionic eminence.23 5HT3A interneurons constitute approximately 30% of cortical interneurons and are especially enriched in cortical layers 2/3.21 These interneurons regulate neural activities during cognitive and emotional brain processes, including spatial memory, fear and anxiety.24 In addition, cortical 5HT3A interneurons modulate neurovasculature by inducing vasodilation mediated by nitric oxide (NO) and vasoconstriction mediated by neuropeptide Y (NPY).25
5HT3A interneurons are functionally and molecularly heterogeneous and comprise several subclasses with distinct roles in cortical circuits. Key 5HT3A interneuron subclasses include:
Figure 9: IHC of mouse brain tissue stained with Anti-5HT3A Receptor Antibody (A11858).
Figure 10: IHC of human brain tissue stained with Anti-NPY Antibody (A98310).
Glycinergic interneurons are specialized inhibitory neurons that release glycine to mediate chlorine-dependent inhibition. These neurons coordinate motor reflexes, sensory processing and network synchronization in the spinal cord and brain stem.29,30 The characterization of glycinergic interneurons mainly relies on specific markers that label the structural and functional components of glycinergic neurotransmission.
GLYT2 and VGAT
In the spinal cord, three principal glycinergic interneuron types have been described. Renshaw cells are located in the ventral horn and are well known for providing recurrent inhibition to motor neurons. This inhibition is mediated by large synaptic terminals expressing the membrane-bound glycine transporter 2 (GLYT2), which are a hallmark of glycinergic neurotransmission.31 Another marker, the vesicular GABA transporter (VGAT), also known as VIAAT and SLC32A1, which transports both GABA and glycine into synaptic vesicles, needs to be co-expressed with GLYT2 in a neuron to fulfil glycinergic transmission.31
Glycine receptors (GlyRs) and Gephyrin
At the postsynaptic level, glycinergic signaling is mediated by glycine receptors (GlyRs), which are pentameric ligand-gated chloride channels constituting α (α1-α4) and β subunits. The α1 subunit (GlyRα1) is the most abundant in adult spinal cord and brainstem neurons, making it a useful marker for glycinergic synapses.32,33 Gephyrin (GPHN), a scaffolding protein that anchors GlyRs at inhibitory synapses, is another valuable marker.29
All the aforementioned glycinergic markers can also be used to identify dorsal horn interneurons as well as ventral inhibitory commissural interneurons. Dorsal horn interneurons are primarily found in laminae I-III of the spinal cord, responsible for gating nociceptive (pain) and pruritic (itch) signals,34 while ventral inhibitory commissural interneuron project glycinergic axons across the midline to regulate timing and synchronization of motor activity on both sides of the body.35
mGluR2 and neurogranin
In the brain stem, 65% of Golgi cells located in the cerebellar granule layer are capable of co-releasing glycine and GABA. They can be identified by their co-expression of molecular markers such as mGluR2 and neurogranin.36 These features allow Golgi cells to modulate granule cell activity and contribute to the fine-tuning of cerebellar processing. On the other hand, Lugaro and globular cells represent other cerebellar interneuron classes that are also glycinergic and GABAergic and can be distinguished by their lack of mGluR2 expression.36 They play a role in regulating circuits within the molecular layer of the cerebellum, further highlighting the functional heterogeneity among glycinergic interneurons.36,37
Glutamatergic interneurons are a small but functionally important excitatory neuron population in the nervous system. While most excitatory glutamatergic neurons are long-range projection neurons, glutamatergic interneurons synapse locally and primarily modulate microcircuits in the spinal cord and intestine.38,39 These neurons release glutamate as their primary neurotransmitter and play crucial roles in regulating network excitability, synaptic plasticity and rhythmic activity.40
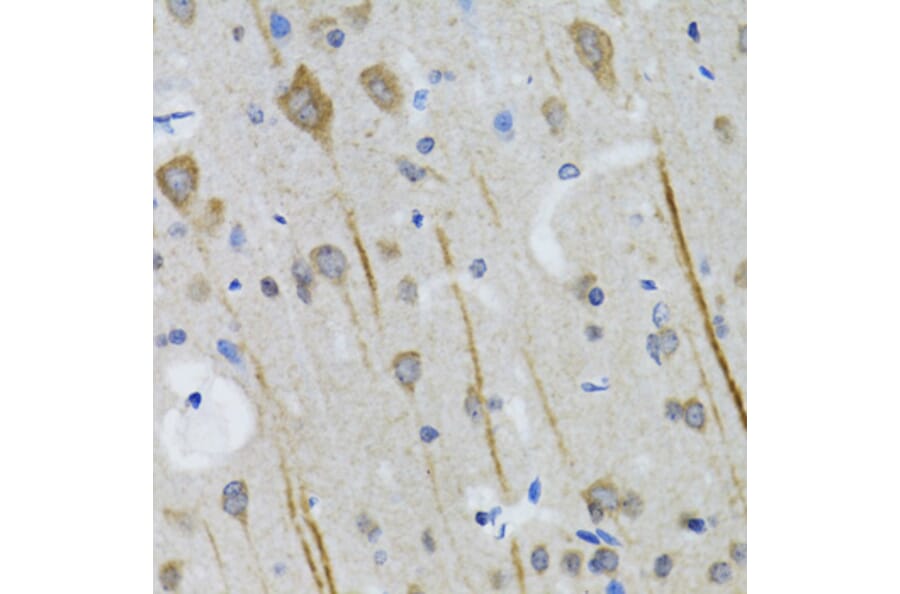
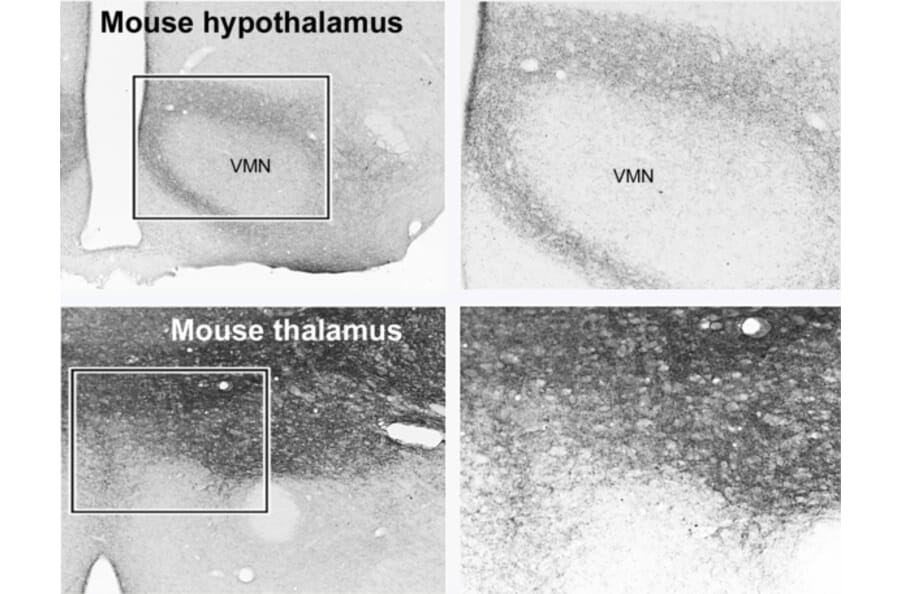
Glutamatergic interneuron classes include SIM1-expressing spinal V3 interneurons that drive locomotor rhythms,39 and enteric glutamatergic interneurons that regulate intestinal motility in the small intestine and colon.38 In addition to SIM1, these glutamatergic interneuron classes can be characterized using general glutamatergic markers as described in the Glutamatergic Neuron Markers page; for instance, enteric glutamatergic interneurons can be identified by VGLUT2.38
Figure 11: IHC of mouse brain tissue stained with anti-VGluT1 Antibody (A326310).
Figure 12: IHC of mouse brain tissue stained with anti-VGLUT2 Antibody (A90888) in red. Nuclei are marked by DAPI in blue.


![IHC - Anti-Parvalbumin Antibody [3C9] (A85317)](https://cdn.antibodies.com/image/catalog/85/A85317_2.jpg?profile=product_image)

![IHC - Anti-Somatostatin Antibody [ARC50670] (A308885)](https://cdn.antibodies.com/image/catalog/308/A308885_3.jpg?profile=product_image)

![IHC - Anti-Calretinin Antibody [3G9] (A85367)](https://cdn.antibodies.com/image/catalog/85/A85367_1.jpg?profile=product_image)
![IHC - Anti-Calbindin Antibody [5A9] (A85362)](https://cdn.antibodies.com/image/catalog/85/A85362_1.jpg?profile=product_image)

![ICC/IF of SH-SY5Y cells using Anti-VAChT Antibody [S6-38] (A305008)](https://cdn.antibodies.com/image/category/primaries/cell-markers/Glutamatergic_IHC_A90888_3_crop.jpg)