Western blotting (WB) is a laboratory technique used to detect changes in abundance of a specific protein or of a post-translational protein modification. The western blotting process involves multiple steps that may introduce procedural, rather than experimental or biological variation, due to differences introduced during one or more of the steps in the process. Such variation includes unequal sample extraction/preparation, variation in protein sample loading, poor/unequal separation during electrophoresis, unequal transfer to a membrane support, poor/patchy incubation with primary or secondary antibodies, or unequal signal detection.
These errors can be reduced, or corrected for, by co-detecting appropriate loading control proteins which serve as an internal reference in western blot experiments. The loading control(s) used depend both on the protein or protein modification being studied (particularly its abundance or sub-cellular localization) and the experimental samples used (e.g. samples on the same immunoblot derived from different tissues or species).
In general, loading control proteins are usually expressed at stable levels between the samples of interest and usually conform to the following criteria:
The table below shows the most common loading controls for each sample type.
| Sample Type | Loading Control | Molecular Weight (kDa) * |
|---|---|---|
| Cytoskeleton | Actin | 42 |
| beta Actin | 42 | |
| Cofilin | 18 | |
| alpha Tubulin | 50 | |
| beta Tubulin | 50 | |
| Membrane | Sodium Potassium ATPase | 110 |
| Mitochondrial | COX IV | 17 |
| Heat Shock Protein 60 | 60 | |
| VDAC1 | 31 | |
| Serum | Transferrin | 77 |
| Nuclear | HDAC1 | 55 |
| Histone H3 | 15 | |
| Lamin B1 | 66 | |
| PCNA | 29 | |
| TBP | 38 | |
| YY1 | 45 | |
| Whole Cell | Cyclophilin B | 21 |
| GAPDH | 37 | |
| Vinculin | 124 |
* The exact molecular weight may vary between species.
Figure 1: Western blot analysis of tissue and cell lysates using Anti-UCHL1 Antibody (A85349) (green). The lanes contain: (1) protein standard, (2) rat brain, (3) mouse brain, (4) NIH-3T3, (5) HEK293, (6) HeLa, (7) SH-SY5Y. Anti-Actin Antibody (A85388) (red) was used as a loading control.
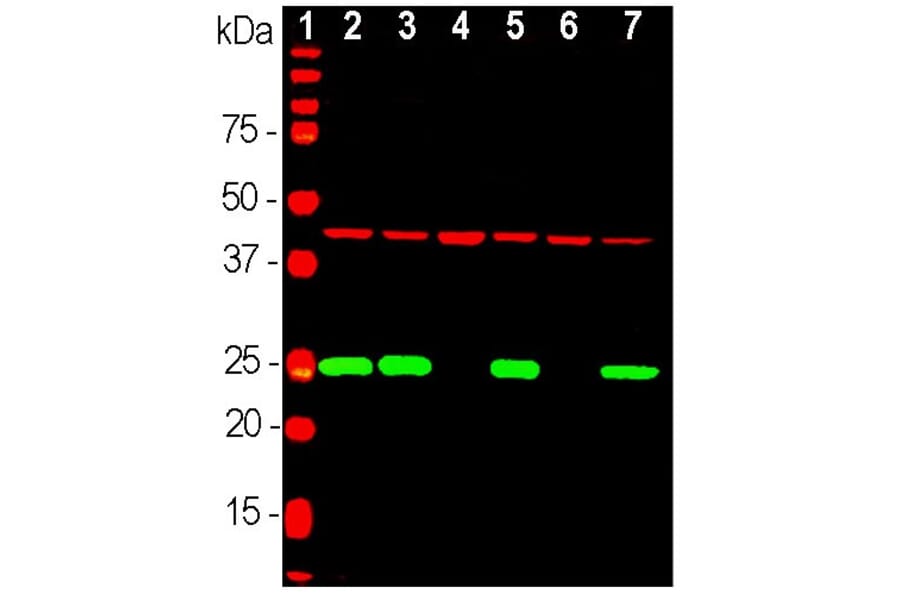
Figure 2: Western blot analysis of cell lysates using Anti-GAPDH Antibody [1D4] (A85382) at a dilution of 1:2,000. The lanes contain: (1) protein standard, (2) HEK293, (3) HeLa, (4) SH-SY5Y, (5) COS1, (6) NIH-3T3, and (7) C6 cell lysates.
Actin
Actins are a family of six proteins in three groups in human and other vertebrates: alpha cardiac muscle 1 alpha 1 (skeletal muscle) and 2 (aortic smooth muscle), beta, gamma 1 and gamma 2 (enteric smooth muscle). Beta and gamma 1 are two non-muscle actin proteins. They function as the main components of actin-based microfilaments. -actin remains one of the most widely used of loading controls due to its high level and relatively stable expression. However, its use as a universal loading control has been questioned 9 and other less abundant actin binding proteins such as cofilin may well be a good alternative choice when considering a cytoskeletal loading control protein 10 (e.g. Anti-Cofilin Antibody (A96586), Anti-Cofilin Antibody (A27001)).
| Recommended Antibodies | Validated Reactivity |
|---|---|
| Anti-HA Tag Antibody (A121677) | WB |
| Anti-HA Tag Antibody (HA.C5) (A85278) | Dot Blot, ELISA, IP, WB |
| Anti-HA Tag Antibody (16.43) (A250863) | ELISA, IF, WB, IP, Flow Cytometry, IHC-P |
| Target | Antibody | Reactivity |
|---|---|---|
| Sodium Potassium ATPase | Anti-ATPase Antibody (A95104) | Human, Mouse, Rat |
| Anti-ATPase Antibody (A92479) | Human, Mouse, Rat |
| Target | Antibody | Reactivity |
|---|---|---|
| COX IV | Anti-COX IV Antibody (A82550) | Human |
| Anti-COX IV Antibody (A9916) | Human, Mouse, Rat | |
| Heat Shock Protein 60 | Anti-Heat Shock Protein 60 Antibody (A85438) | Human, Mouse, Rat |
| Anti-Heat Shock Protein 60 Antibody (A85437) | Human, Mouse, Rat | |
| Anti-Heat Shock Protein 60 Antibody (1C7) (A85439) | Human, Mouse, Rat | |
| VDAC1 | Anti-VDAC1 Antibody (A11230) | Human, Mouse |
| Target | Antibody | Reactivity |
|---|---|---|
| HDAC1 | Anti-HDAC1 Antibody (A12564) | Human, Mouse, Rat |
| Anti-HDAC1 Antibody (A84192) | Human, Mouse | |
| Histone H3 | Anti-Histone H3 Antibody (A25203) | Human, Mouse, Rat |
| Anti-Histone H3 Antibody (A16702) | Human, Mouse, Rat | |
| Anti-Histone H3 Antibody (RM186) (A121358) | All Vertebrates | |
| Anti-Histone H3 Antibody (RM190) (A121350) | All Vertebrates | |
| Lamin B1 | Anti-Lamin B1 Antibody (A82838) | Human, Mouse |
| Anti-Lamin B1 Antibody (A26974) | Human, Mouse, Rat | |
| PCNA | Anti-PCNA Antibody (A83563) | Human, Mouse, Rat, Porcine |
| Anti-PCNA Antibody (A25315) | Human, Mouse, Rat | |
| Anti-PCNA Antibody (PC10) (A86878) | Human, Mouse, Rat, Chicken, Drosophila | |
| TBP | Anti-PCNA Antibody (A26155) | Human, Mouse, Rat |
| Anti-PCNA Antibody (A82888) | Human, Mouse, Rat | |
| YY1 | Anti-YY1 Antibody (A8486) | Human, Mouse |
| Target | Antibody | Reactivity |
|---|---|---|
| Transferrin | Anti-Transferrin Antibody (HTF-14) (A85619) | Human, Rabbit, Porcine |
| Anti-Transferrin Antibody (PTF-02) (A85822) | Porcine |
| Target | Antibody | Reactivity |
|---|---|---|
| Cyclophilin B | Anti-Cyclophilin B Antibody (Pk2E2AT) (A58461) | Human |
| GAPDH | Anti-GAPDH Antibody (A83722) | Human, Mouse, Rat |
| Anti-GAPDH Antibody (A85377) | Human, Horse, Cow, Porcine, Chicken, Rat, Mouse | |
| Anti-GAPDH Antibody (1D4) (A85382) | Human, Horse, Cow, Porcine, Chicken, Rat, Mouse | |
| Anti-GAPDH Antibody (GA1R) (A85271) | Chicken, Hamster, Human, Mouse, Rat, Rabbit, Sf9 Insect, BL-21 Bacteria, S. cerevisiae | |
| Anti-GAPDH Antibody (RM114) (A121396) | Human, Monkey | |
| Vinculin | Anti-Vinculin Antibody (A94871) | Human, Mouse, Rat |
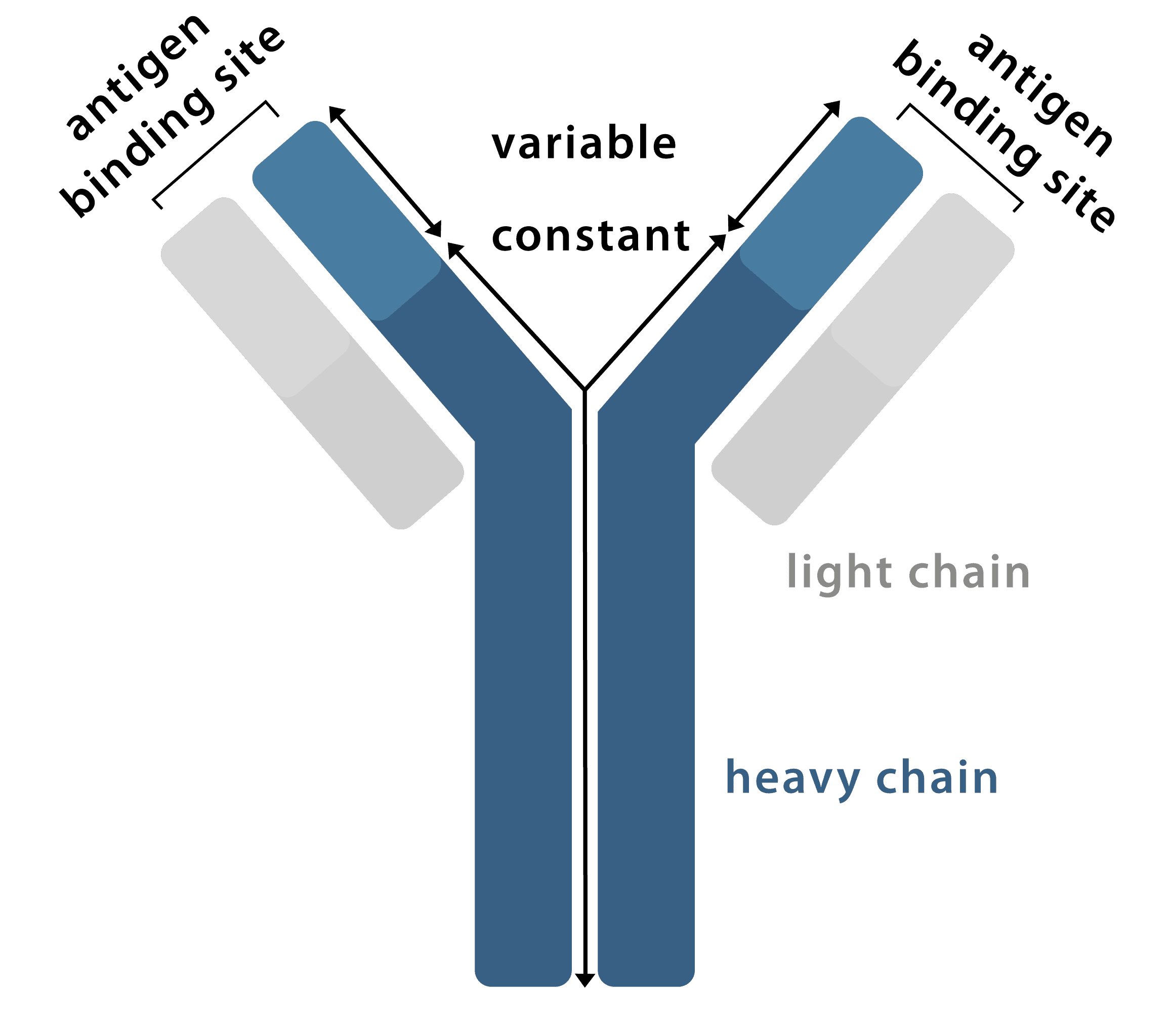
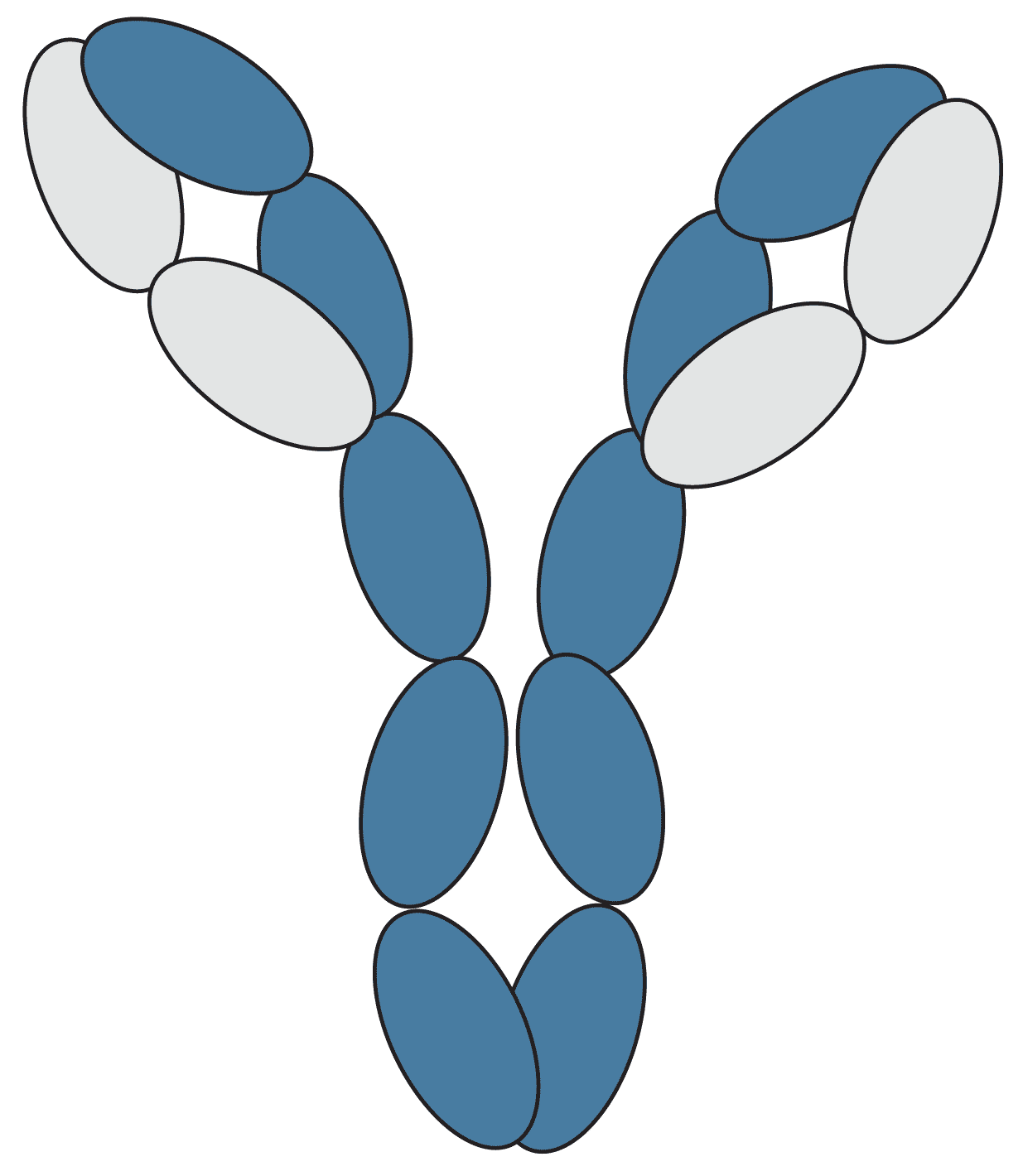
An antibody is a "Y-shaped" glycoprotein, produced by the body's immune system in response to invasion by foreign molecules, that is capable of binding to specific antigens. Each antibody is composed of four polypeptide chains, two identical heavy chains and two identical light chains, which vary in sequence and length. An antibody can be broken down into two F(ab) regions, the top sections of the "Y" which contain the variable region which binds specifically to a particular epitope on the antigen, and an Fc region, the bottom of the "Y" which provides a binding site for endogenous Fc receptors (and secondary antibodies).
Figure 1: Graphical representation of an antibody.
Monoclonal antibodies are produced by injecting an antigen into a host animal in order to initiate a humoral immune response, and by subsequently extracting the antibody-producing spleen cells and fusing them in vitro with cultured malignant myeloma cells to create immortal hybridoma cell lines. This provides a stable, long-term supply of monoclonal antibodies. Monoclonal antibodies are identical, recognizing a single epitope, and are derived from a group of identical cloned cells. A clone ID is given to each clone of hybridoma cells which identifies the monoclonal antibody it produces.
Polyclonal antibodies are produced by injecting an antigen into a host animal in order to initiate a humoral immune response. After the initial immunisation, the host animal will receive a secondary, and potentially a tertiary, immunisation in order to produce higher concentrations of antibodies against the particular antigen. Polyclonal antibodies are a collection of multiple different antibodies, derived from the immune response of multiple B-cells, which each recognize a different epitope on the same antigen.
Monoclonal vs Polyclonal Antibodies
| Polyclonal | Monoclonal |
|---|---|
| A heterogeneous antibody population. | A homogenous antibody population. |
| Lack of epitope specificity; will bind to multiple different epitopes on an antigen. | High specificity and affinity for a single epitope on a single antigen. |
| Increased likelihood of cross-reactivity with similar antigens. | Lower risk of cross-reactivity (due to specificity for a single epitope). |
| High batch-to-batch variability. | No / low batch-to-batch variability. |
| Can create background noise in some applications. | Create less background noise from staining of sections / cells. |
| Relatively inexpensive to produce. | Significantly more expensive to produce. |
| Relatively quick to produce (~ 3 months). | Slow to produce (~ 6 months). |
In mammals, antibodies are classified into five main classes or isotypes according to the heavy chain they contain. These are: IgA (alpha), IgD (delta), IgE (epsilon), IgG (gamma), and IgM (mu). Each class differs in: the sequence of constant domains, the number of constant domains, the hinge structure, and the valency of the antibody.
The light chains of an antibody are classified as either kappa or lambda based on their polypeptide sequence. Typically, only one type of light chain is present in an individual antibody and, as such, the two light chains are identical.
| Isotype | Heavy Chain | Light Chain | Structure |
|---|---|---|---|
| IgG1 | γ1 | λ or κ | Monomer |
| IgG2a | γ2 | λ or κ | Monomer |
| IgG2b | γ2 | λ or κ | Monomer |
| IgG3 | γ3 | λ or κ | Monomer |
| IgG4 | γ4 | λ or κ | Monomer |
| IgA1 | α1 | λ or κ | Monomer or Dimer |
| IgA2 | α2 | λ or κ | Monomer or Dimer |
| IgD | δ | λ or κ | Monomer |
| IgE | ε | λ or κ | Monomer |
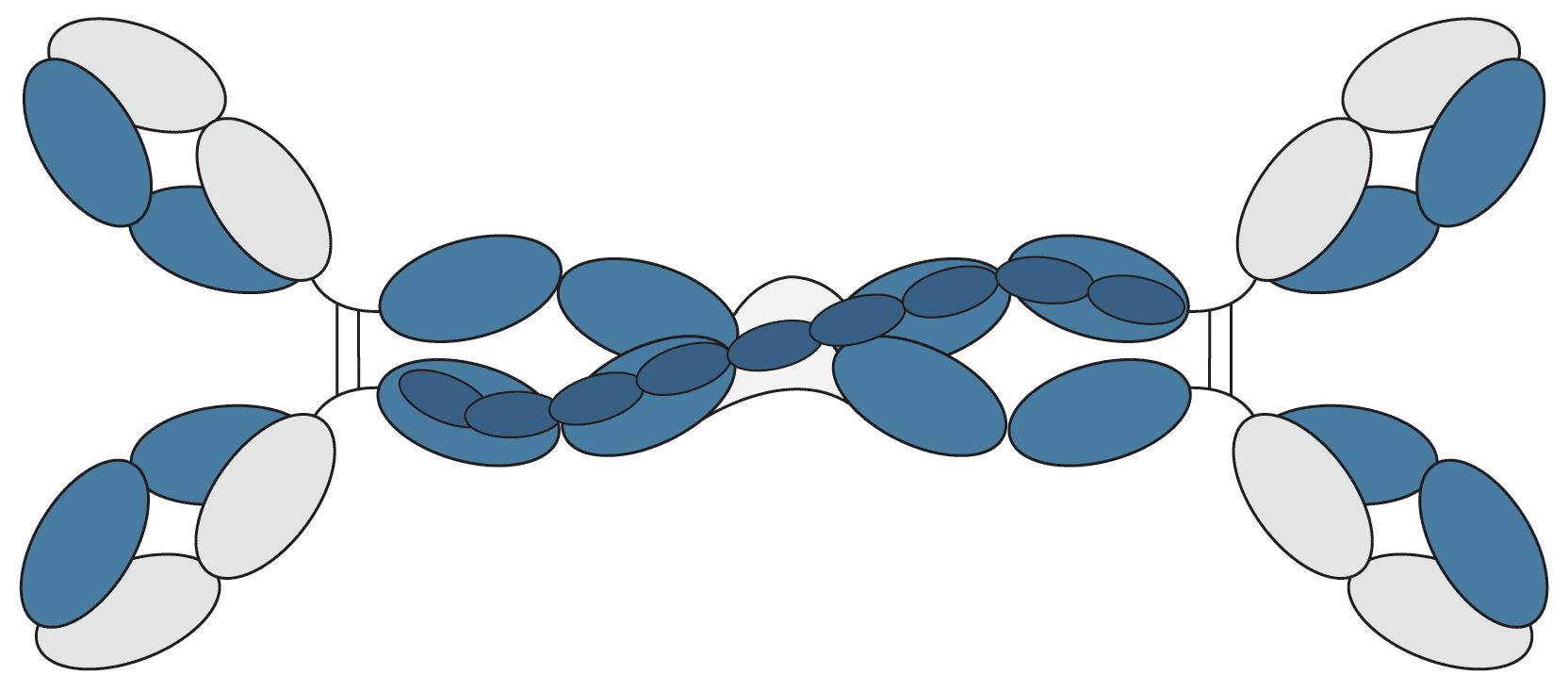
| IgM | μ | λ or κ | Pentamer |
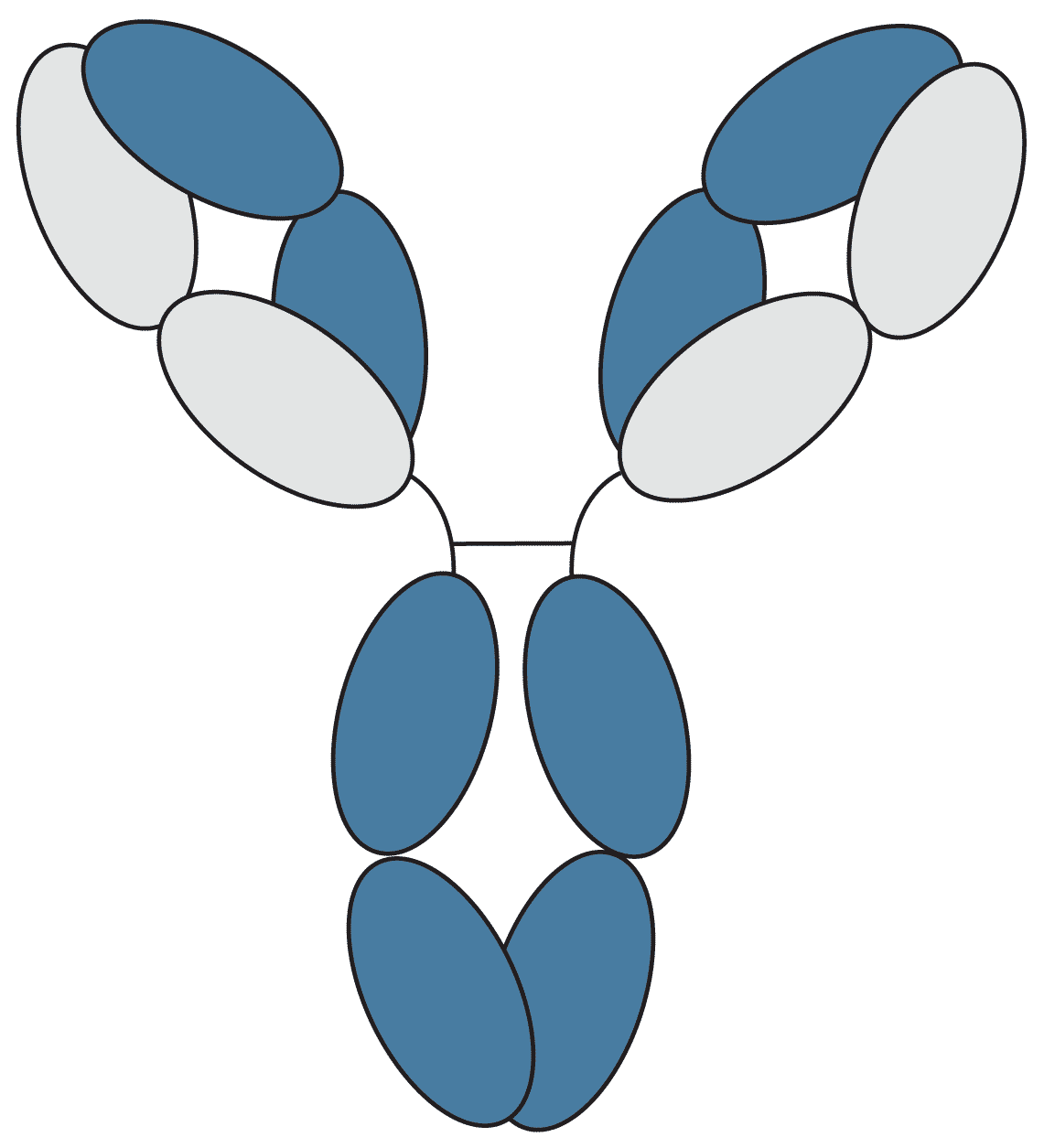
IgG (consisting of IgG1, IgG2a, IgG2b, IgG3, and IgG4) is the most abundant antibody in normal human serum, accounting for 70-85% of the total immunoglobulin pool. IgG is involved in placental transfer, with the mother’s IgG transferring to the foetus in order to provide immune protection during the initial stages of development.
Figure 2: Graphical representation of IgG.
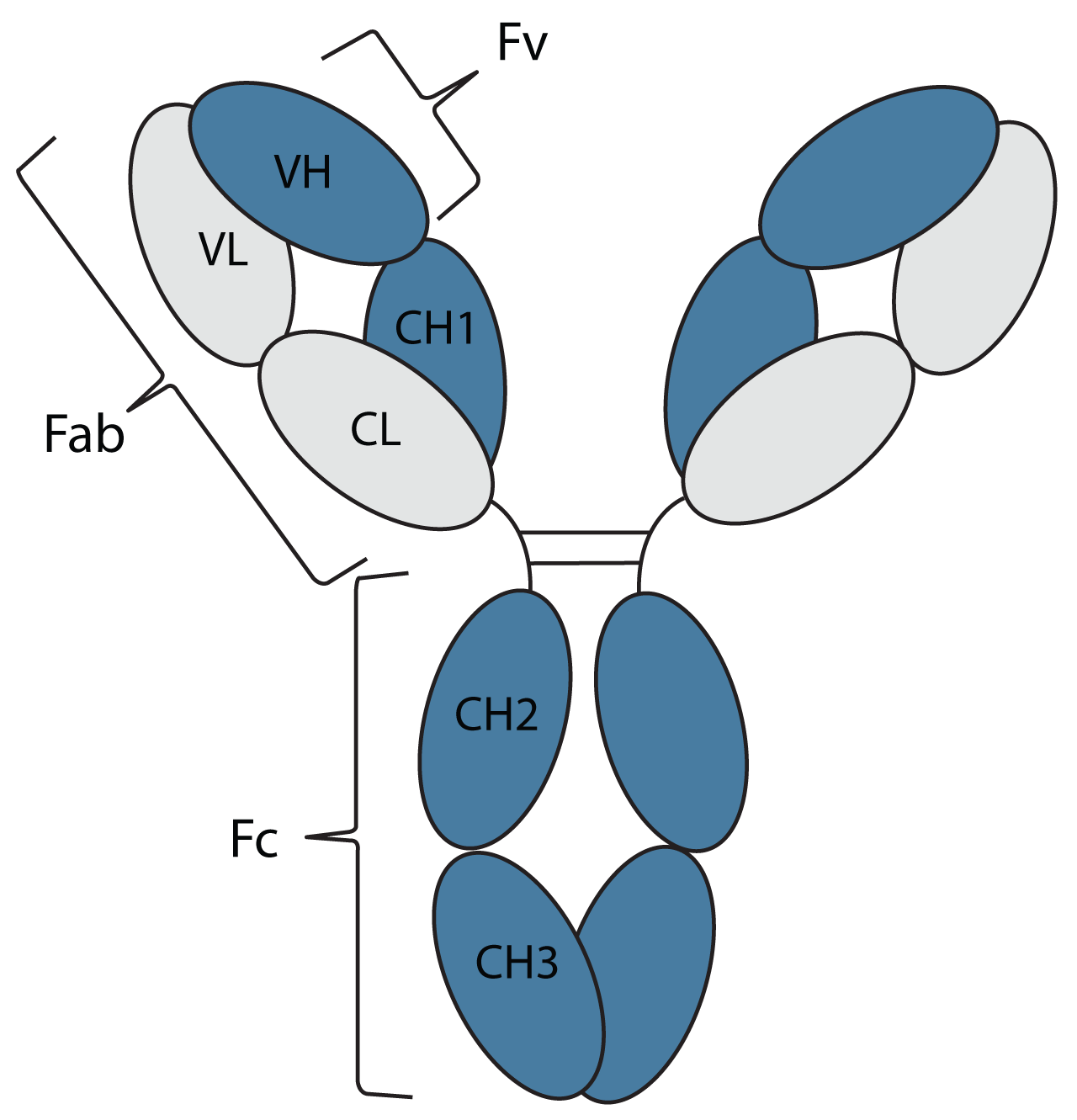
Each oval represents a protein domain in the antibody. These are systematically labelled by their chain type (L = Light chain or H = Heavy chain) and whether their sequence changes to bind an antigen (V = Variable domain or C = Constant domain), with CH domains numbered from the variable region. The domains are joined by linker regions of protein and disulfide bonds, represented by lines.
The antibody's structure is also described in terms of the fragments made by protein degradation or enzymatic cleavage of linker regions, namely the antigen-binding fragments (Fab), the variable fragments (Fv), and the constant fragment (Fc).
IgA (consisting of IgA1 and IgA2) accounts for around 5-15% of the antibody pool, existing either as a monomer or a dimer. IgA is the predominant antibody in mucous secretions, such as saliva, tears, and milk.
Figure 3: Graphical representation of IgA.
IgD only accounts for approximately 1% of the total plasma immunoglobulin, however, it is found in large quantities on the membrane of B-cells. IgD has the same basic structure as IgG but has an extended hinge region, which is susceptible to proteolytic digestion. IgD is thought to serve as an antigen receptor for the activation of B cells.
Figure 4: Graphical representation of IgD.
IgE only accounts for approximately 0.002% of serum antibodies and is found on the basophils and mast-cells. Similar to IgM, IgE has two additional constant domains instead of a hinge region. IgE is thought to play a role in immunity to parasites but is more commonly associated with type I immediate hypersensitivity, responding to innocuous environmental antigens such as pollen and peanuts, which can result in extreme reactions such as anaphylaxis or allergic rhinoconjunctivitis.
Figure 5: Graphical representation of IgE.
IgM accounts for between 5-10% of the immunoglobulin population and is the predominant antibody in the body's primary immune response. It is often represented as a pentamer, with a five-chain structure held together by a J chain, however, it can also exist in a hexameric form, without the J chain, and as a monomer on the surface of B-cells. The large size of soluble IgM (~ 900kDa) mainly confines this antibody to the intravascular pool.
Figure 6: Graphical representation of IgM.
Each clone number represents a specific cell line cloned from ascites that was used to manufacture the antibody. Since monoclonal antibodies are produced by more than one host, and more than one cell line, each cloned cell line receives a unique clone number to identify it.
Secondary antibodies should be raised against the host species of the primary antibody you are using. For example, if your primary antibody is a rat monoclonal you will require an anti-rat secondary antibody. We recommend checking the datasheet of the secondary antibody to ensure that it has been validated in the application you will be using.
Isotype controls are used to confirm that the binding of the primary antibody is specific and not a result of other protein interactions or non-specific Fc receptor binding.
The isotype control antibody should match the primary antibodies host species, isotype, and conjugation. For example, if the primary antibody is a HRP-conjugated rat IgG1, then you will need a HRP-conjugated rat IgG1 isotype control.
We recommend checking the datasheet first, which will often have a suggested positive control. It is important to ensure that the tissue or cell line used is from a tested species.
If no positive controls are suggested, we recommend looking at the UniProt website. This database often has a list of tissues that the protein is expressed in. These tissues can be considered suitable positive controls.
![Western Blot - Anti-GAPDH Antibody [1D4] (A85382) - Antibodies.com](https://cdn.antibodies.com/image/catalog/85/A85382_2.jpg?profile=product_image)