Ryan Hamnett, PhD | 10th October 2025
G protein-coupled receptors (GPCRs) are a large superfamily of cell-surface receptors representing the targets of approximately one third of all approved drugs.1 Characterized by their highly conserved structure of 7 transmembrane (7TM) helices, GPCRs respond to a wide variety of extracellular signals such as photons, hormones, neurotransmitters and odorants, and have critical roles in mediating a diverse array of cellular processes. Agonist exposure triggers GPCR phosphorylation on their intracellular C-terminal tail, and the precise nature of this phosphorylation is an essential regulator of their function, determining downstream signaling, desensitization, internalization, and interactions with proteins such as β-arrestin.2
GPCRs are technically demanding to work with due to their low natural abundance and their dependence on a membrane environment for stability. The 7TM structure of GPCRs creates a propensity to aggregate, while phosphorylation of GPCRs is extremely site-specific and highly transient. These issues necessitate highly validated antibodies against GPCRs and specific phosphorylated residues, as well as optimized and robust protocols to detect GPCRs by western blot, IHC and ICC/IF. These resources are available directly through antibodies.com and are described below.
For tools beyond antibodies that can be used to investigate GPCRs and other membrane proteins, see our Transmembrane Proteins and Nanodiscs pages.
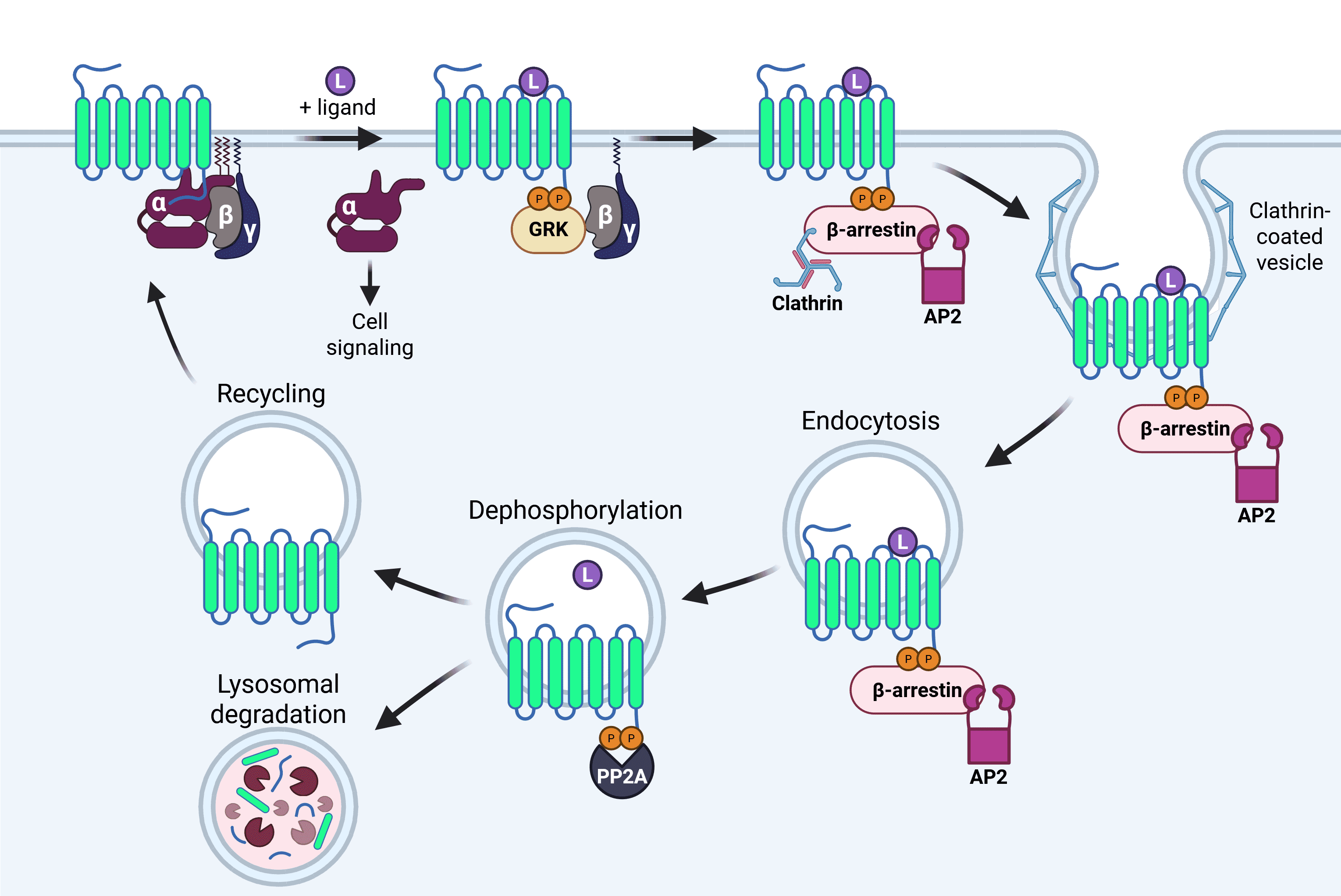
Phosphorylation of GPCRs occurs on intracellular serine and threonine residues on the C-terminal tail that are unmasked due to conformational changes following agonist exposure.3 These structural changes are recognized by highly specific GPCR kinases (GRKs), as well as more general kinases such as protein kinase A (PKA). Ligand-mediated phosphorylation regulates acute receptor desensitization, arrestin recruitment, internalization, post-activation signaling and, in some cases, long-term tolerance to the agonist (Figure 1).4
Figure 1: Agonist-mediated GPCR internalization. Ligand binding to the GPCR results in conformational changes that are recognized by GPCR receptor kinases (GRKs). GRKs result in phosphorylation of the C-terminal tail, increasing affinity for arrestins. Arrestin binding leads to the dissociation of the heterotrimeric G protein from the GPCR and targeting to clathrin-coated pits for endocytosis with the help of the AP2 protein. Once internalized, the ligand dissociates and is typically degraded, and the GPCR is dephosphorylated by a protein phosphatase such as PP2A. The receptor can then be recycled to the plasma membrane or trafficked to lysosomes for degradation. Image made in BioRender.
Most GPCRs have multiple sites for phosphorylation, which are often not conserved, and the unique combination of phosphorylated sites, or the phosphorylation ‘barcode’, is important in determining the downstream effects and responses to stimuli.3 For example, the C-terminal tail of the mu opioid receptor has at least 4 distinct phosphorylation sites (Thr370, Ser375, Thr376, Thr379), which show differential responses to opioid stimulation.5 Antibodies against phosphorylated GPCRs must therefore be site-specific and highly validated to avoid cross-reactivity.
Our phosphosite-specific GPCR antibodies are designed to specifically detect agonist-activated GPCRs, using the relevant phospho-peptide as the immunogen.
Western blot is a useful technique for measuring changes in GPCR expression or the level of phosphorylation in response to pharmacological treatments. The following tips will increase the chance of developing a strong, reliable signal.
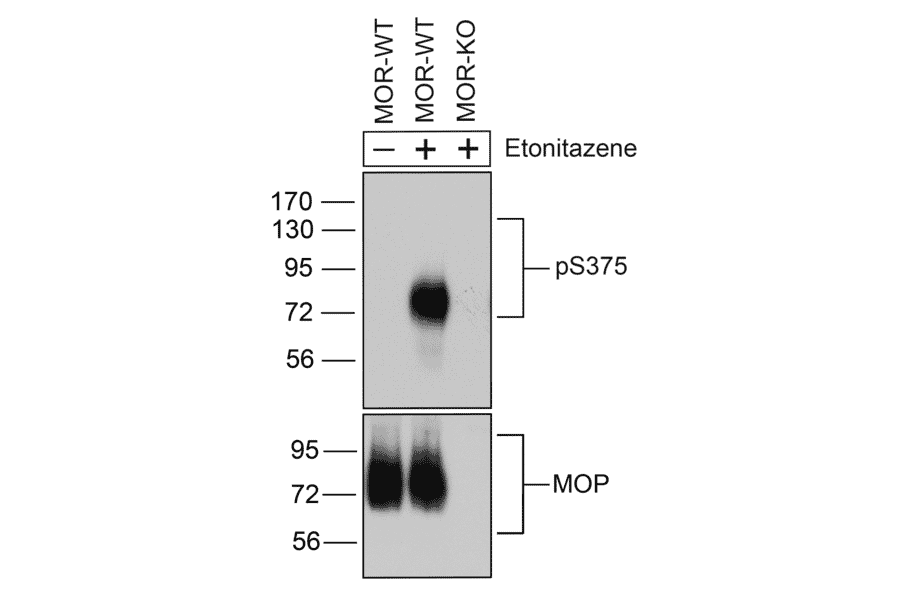
Figure 2: Western blot of wild type and mu opioid receptor (MOR) knockout mouse brain tissue lysate stained with Anti-Mu Opioid Receptor (phospho Ser375) Antibody (A334520). Treatment with etonitazene triggers MOR phosphorylation.
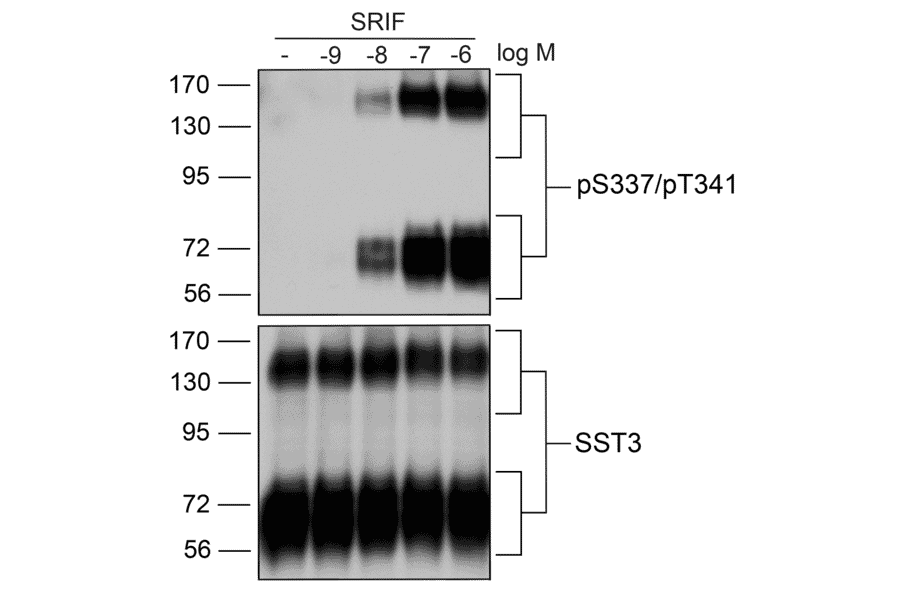
Figure 3: Western blot of HEK293 cell lysate stained with Anti-SSTR3 (phospho Ser337 + Thr341) Antibody (A334544), demonstrating dose-dependent phosphorylation of SSTR3 by SRIF.
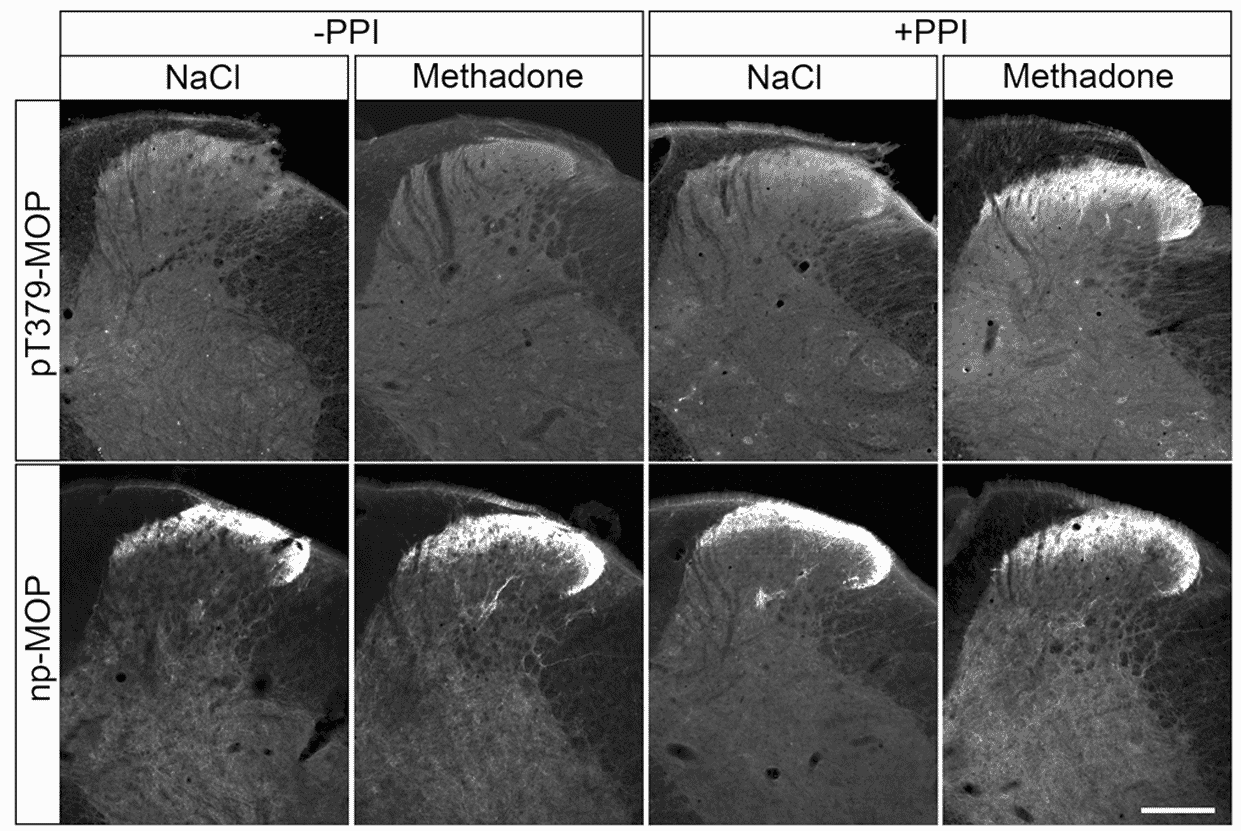
While IHC or ICC/IF for GPCRs is commonly done (and typically doesn’t suffer from the issues described above for western blots), performing these techniques on phospho-GPCRs can be much more challenging due to the transience of the phosphorylation. Endogenous phosphatases result in dephosphorylation being lost during sample preparation. The simple solution to this is to add a phosphatase inhibitor during the protocol to maintain phosphorylation state (Figure 4).5
See our Immunohistochemistry Protocols and ICC/IF Protocol for step-by-step instructions for performing these techniques.
Figure 4: Effect of phosphatase inhibitors on phospho-GPCR IHC results. IHC of non-phosphorylated (np) or Thr379 phosphorylated (pT379) mu opioid receptor (MOP) following treatment with methadone in the presence or absence of a protein phosphatase inhibitor (PPI). Signal is only observed in the presence of PPI. Reproduced under CC BY 4.0 from 5
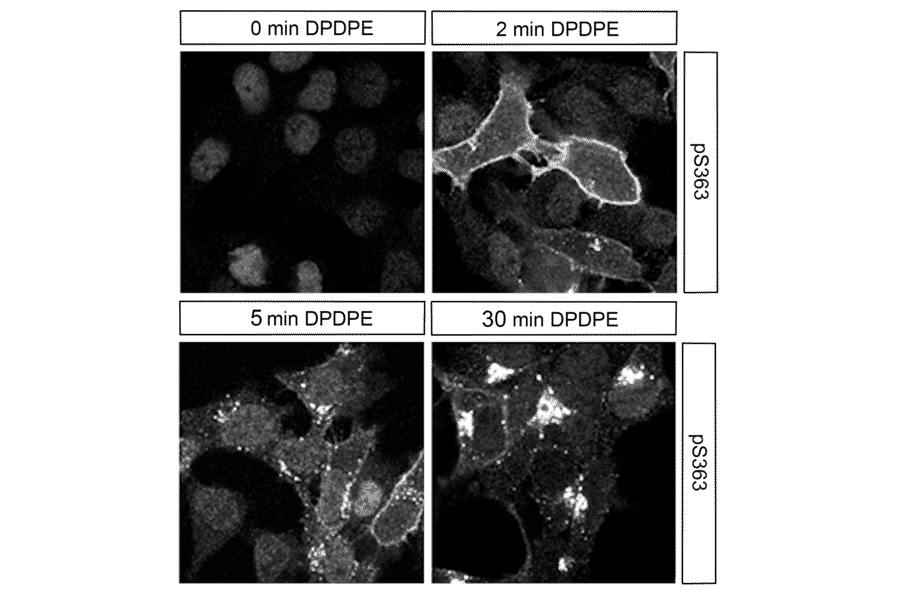
Figure 5: ICC/IF of HEK293 cells stably expressing delta opioid receptor (DOR) stained with Anti-delta Opioid Receptor (phospho Ser363) Antibody (A334519). Treatment with DPDPE triggers DOR phosphorylation and internalization.
Figure 6: ICC/IF of HEK293 cells stably expressing Somatostatin Receptor 2 (SSTR2) stained with Anti-SSTR2 (phospho Thr356 + Thr359) Antibody (A334541). Treatment with SRIF triggers phosphorylation of SSTR2.
Antibodies are an essential tool for characterizing GPCR function and responses to pharmacological manipulation, which are important to understand for the development of new therapeutics, given that GPCRs represent approximately one third of FDA-approved drugs.1
Phosphosite-specific antibodies are novel tools for GPCR research that can be used to:
| Antibody | Reactivity | Applications |
|---|---|---|
| Anti-CXCR4 (phospho Ser324 + Ser325) Antibody | Human, Mouse | WB |
| Anti-CXCR4 (phospho Ser346 + Ser347) Antibody | Human, Mouse | WB |
| Anti-delta Opioid Receptor (phospho Ser363) Antibody | Human, Mouse, Rat | WB, ICC/IF |
| Anti-Mu Opioid Receptor (phospho Ser375) Antibody | Human, Mouse, Rat | WB, ICC/IF |
| Anti-Mu Opioid Receptor (phospho Ser375) Antibody | Human, Mouse, Rat | WB, ICC/IF, IHC |
| Anti-Mu Opioid Receptor (phospho Thr370) Antibody | Human, Mouse, Rat | WB |
| Anti-Mu Opioid Receptor (phospho Thr376) Antibody | Human, Mouse, Rat | WB |
| Anti-SSTR2 (phospho Ser341 + Ser343) Antibody | Human, Mouse | WB |
| Anti-SSTR2 (phospho Thr356 + Thr359) Antibody | Human, Mouse | WB, ICC/IF |
| Anti-SSTR3 (phospho Ser337 + Thr341) Antibody | Human | WB, ICC/IF |
| Anti-SSTR5 (phospho Thr333) Antibody | Human | WB |