Heather Van Epps, PhD | 20th March 2025
Regulatory T (Treg) cells are a type of CD4+ T cell that play an essential role in maintaining peripheral tolerance by modulating the function of other immune cells, including CD4+ T helper (Th) cells, cytotoxic CD8+ T cells, B cells and dendritic cells.1 Treg cells are required to dampen or repress excessive immune responses, to promote and sustain tolerance to self, commensal, and food antigens, and to maintain tissue homeostasis.2
Fundamental defects in the development of Treg cells result in a lethal systemic autoimmune disease called IPEX syndrome (scurfy in mice),3 and defects in Treg cell function have been described in a variety of chronic inflammatory conditions, including type 1 diabetes, rheumatoid arthritis, and systemic lupus erythematosus.1,3
Various surface molecules can be used to define Treg cells, allowing researchers to enumerate and study these cells in blood and tissues, and investigate their interplay with other immune cell populations. However, the expression of most Treg cell markers by other immune cell populations makes unequivocal identification of these cells challenging. The 2003 discovery of the requirement of the transcription factor FoxP3 for Treg cell lineage commitment in the thymus and suppressive function in the periphery has been fundamental to the study of these cells and allowed for the identification of thymus-derived T cells via intracellular staining and flow cytometry. Treg cells in tissue can also be identified by staining for FoxP3 by IHC (Figure 1, 2).4,5
Like many T cell populations, Treg cells are heterogeneous and functionally diverse, with the activity of these cells dictated by both microenvironmental cues, which trigger transcriptional and epigenetic changes, and their stage of differentiation/activation.1,6,7
The most common markers used to identify Treg cells are CD3, CD4, CD25 (high expression), and CD127 (low expression).3 However, nearly all surface markers of Treg cells are also expressed by activated conventional CD4+ T (Tconv) cells.1
Additional markers are commonly used to identify specific populations of Treg cells (Table 1), including the inhibitory receptor TIGIT, the transcription factor Helios, and the surface glycoprotein GPA33.8
| Cell Type | Cell Surface Markers | Intracellular Markers | Negative Markers |
|---|---|---|---|
| CD4+ Treg | CD3, CD4, CD25high, CD127low, CCR4 | FoxP3 | |
| CD4+ tTreg | CD3, CD4, CD25high, CD127low, TIGIT, GPA33 | FoxP3, Helios | |
| CD4+ pTreg | CD3, CD4, CD25high, CD127low, TIGIT | FoxP3 | Helios, GPA33 |
| CD8+ Treg | CD3, CD8, CD8αα, CD56, CD103, CD122, PD-1, TCRαβ, HLA-E, KIR | CD28 |
Table 1: Expression of regulatory T cell markers in humans. Adapted from 3. For specific combinations of markers used to define cell types, see 3.
Figure 1: Flow cytometry analysis of human peripheral blood cells stained with Anti-FOXP3 Antibody [3G3] (APC) (A86268).
Figure 2: IHC of human tonsil stained with Anti-CD27 Antibody [LPFS2/4177] (A250621).
Treg cells are divided into two broad categories, based on their developmental origins: those that develop in the thymus—called thymic-derived Treg (tTreg) cells (or natural Treg [nTreg] cells)—and those that develop in the periphery—called peripheral-derived Treg (pTreg) cells.
Thymic regulatory T (tTreg) cells
Around 80% of circulating Treg cells develop in the thymus during neonatal development, and these cells are central to the establishment of self-tolerance and prevention of autoimmunity.9 tTreg cells develop from CD4+ single positive thymocytes, and their differentiation into CD25+ Foxp3+ Treg cells requires a tripartite signal: stimulation of the T-cell receptor (TCR) by self-antigen, co-stimulation via CD28, and a third signal downstream of γ-chain cytokines such as interleukin (IL)-2 and IL-15.3,9-11
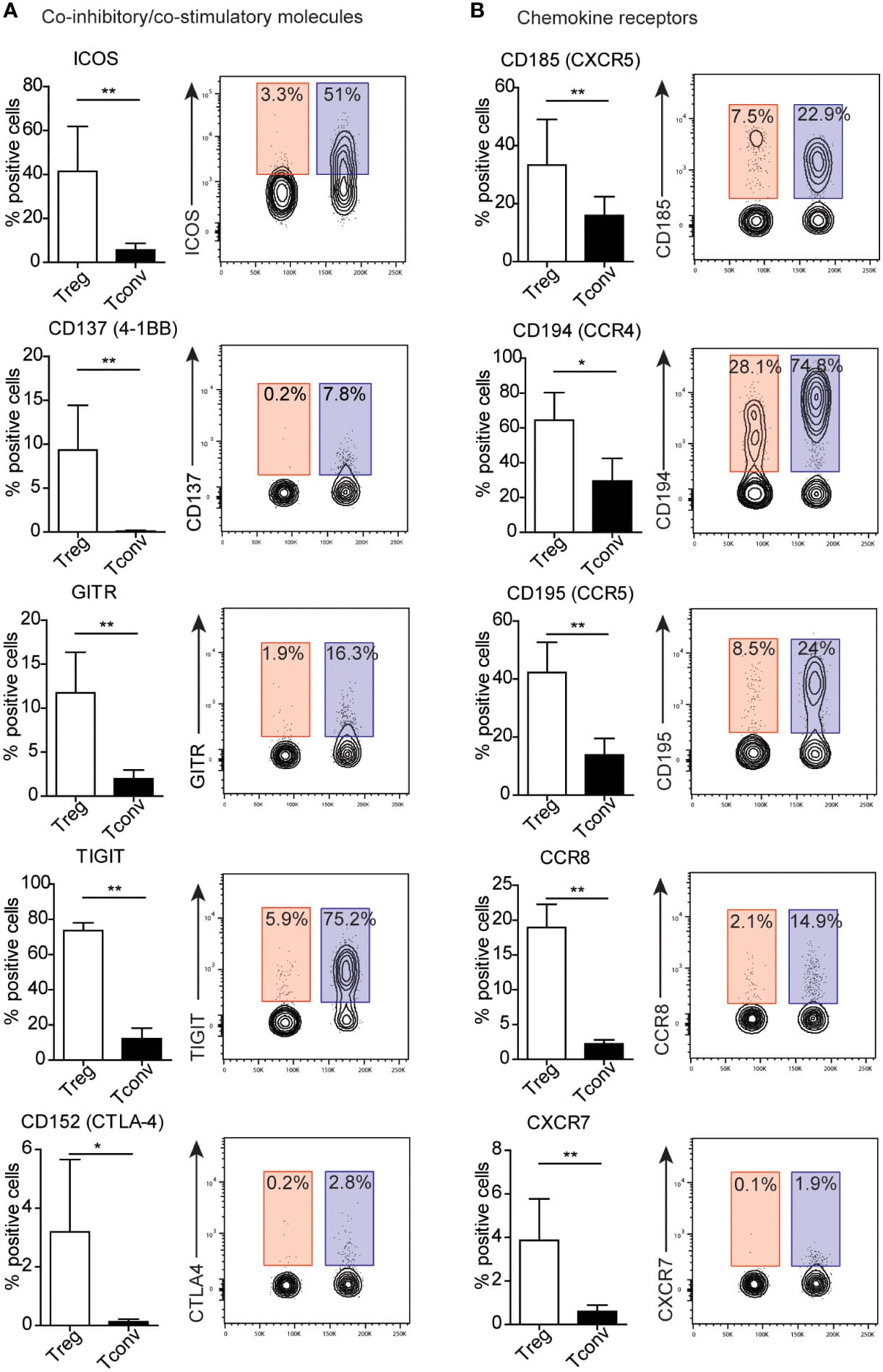
Markers of tTreg cells include the transcription factor Helios (intracellular), neuropilin-1 (Nrp1), and several co-inhibitory receptors including CTLA-4, 4-1BB, ICOS, and GITR. Activated CD4+ effector T cells express the same inhibitory markers, but at lower levels; as such, relative expression levels as measured by flow cytometry is often used to distinguish these cell populations (Figure 3). Most tTreg cells retain stable expression of FoxP3 after leaving the thymus.11
Figure 3: Expression of markers on human peripheral blood mononuclear cells by flow cytometry, comparing resting regulatory T (Treg) cells and conventional T (Tconv) cells.Data is presented both as bar graphs and contour plots. Percentages on the contour plots indicate the percent gated cells as positive for a given surface marker (blue = Treg, red = Tconv). Reproduced under Creative Commons 4.0 CC-BY from 2
Mature tTreg cells can recirculate back to the thymus, where they function to fine-tune the de novo production of tTreg cells, for example via consumption of IL-2.12 Recirculating Treg cells have an activated phenotype (CD44highCD62Llow), and the relative proportion of these cells in the thymus increases with age. Functional and phenotypic heterogeneity exists within recirculating Treg cells, including a population that expresses the IL-1 decoy receptor IL-1R2 and promotes tTreg cell development under inflammatory conditions by quenching IL-1.13
Figure 4: Flow cytometry analysis of Expi293 cells transfected with irrelevant protein (A) or human CTLA-4 (B) stained with Recombinant Anti-CTLA4 Antibody [DM51] (A318645).
Figure 5: IHC of human tonsil stained with Anti-CTLA4 Antibody [IHC064] (A324529).
Peripheral Regulatory T (pTreg) Cells
Peripheral Treg cells arise from conventional CD4+ T cells and convert to FoxP3+ suppressive Treg cells in vivo in response to stimulation of the TCR by sub-immunogenic concentrations of antigen.11 The full array of signals that promote the generation of pTreg cells in vivo are yet to be defined. pTreg cells can be identified by expression FoxP3+CD4+ cells and absence of Helios and Nrp1.6 FoxP3 is not stably expressed in pTreg cells, which exhibit suppressive capacity only when the transcription factor is expressed.3 pTreg cells that lose expression of FoxP3 are known as “exFoxP3” or “exTreg” cells. ExFoxP3 express high levels of CD127, low levels of GITR, and no longer express CD25. Expression of folate receptor 4 (FR4), CTLA-4 and CD103 has also been described on these cells.14
Inducible Regulatory T (iTreg) Cells
Inducible regulatory T (iTreg) cells arise in vitro from tTreg cells in response to stimulation with the cytokines TGF-β and IL-2, which induce and stabilize the expression of FoxP3, respectively.11,15 Although the term iTreg cell is predominantly used to describe in vitro-generated Treg cells, this term is sometimes also applied to cells that are induced to express FoxP3 and gain suppressive function in vivo (i.e. pTreg cells).
Resting (or naïve) tTreg cells require activation via the TCR and other signals to acquire their full suppressive function.
Naïve Treg cells—like naïve conventional T cells—express lower levels of FoxP3 and CD25, CD45RA (the long isoform of CD45) and CD62L.1
Once activated, Treg cells upregulate FoxP3 and CD25, lose expression of CD62L and CD45RA, and gain expression of the shorter isoform of CD45, CD45RO — these cells are variably called activated Treg (aTreg) cells or effector Treg (eTreg cells).11 Also akin to activated conventional T cells, activated Treg cells express a variety of other markers including CD25, CTLA-4, TIGIT, GITR, ICOS, CD44, CD69, CD62L, and CD45RBlow.16 Expression of CD127 has also been shown to increase upon Treg cell activation.17
Figure 6: Flow cytometry analysis of human peripheral blood stained with Anti-CD45RO Antibody [UCHL1] (FITC) (A86178).
Figure 7: IHC of human tonsil stained with Anti-CD45RO Antibody [T200/797] (A249812).
As with resting Treg cells, distinction between activated Treg cells and activated Tconv cells often depends on the relative expression levels of surface markers and the proportion of cells expressing them.2
A third subset of Treg cells—often called non-Treg cells—express low levels of FoxP3 and CD25 and mainly function to promote immune responses via secretion of inflammatory cytokines.11,18 These cells are distinguished from resting Treg cells by the lack of CD45RA expression.
Treg cells are found in many non-lymphoid tissues, including skeletal muscle, skin, intestine, heart, lungs, liver and the central nervous system as well as in lymphoid tissues like germinal centers.6,7 Tissue Treg cells are mostly thymus-derived, but in certain tissues (e.g. intestine), they appear to be primarily peripherally derived.
Subsets of Peripheral Treg Cells
Peripheral Treg cells in tissues that arise from conventional CD4+ T cells adapt to the inflammatory environment in that tissue. This results in Treg cell subpopulations that are tailored to suppress the activity of the predominant local CD4+ helper T (Th) cell population — Th1, Th2, Th17, Th1/17, or follicular T helper (Tfh) cells.1,7
These functionally specialized Treg cell populations mirror the corresponding Th cell population in their transcription factor and cytokine expression profiles. For example, in the context of a predominant Th1 response, such as during viral infection, Treg cells secrete interferon (IFN)-γ and express the transcription factor T-bet, which is critical for their ability to suppress Th1-driven inflammation. These specialized CD4+CD25+FoxP3+ eTreg cell subsets can be distinguished by variable expression of lineage-determining transcription factors and chemokine receptors, as outlined below and in Table 2.1,11
| Subset | Transcription Factors | Phenotype/Markers | Cytokines |
|---|---|---|---|
| Th1-type Treg cells | T-bet | CCR4+ CXCR3+ CCR6- | IFN-γ, TNF-α |
| Th2-type Treg cells | GATA-3, IRF4, STAT6 | CCR4+ CXCR3- CCR6+ | IL-4, IL-5, IL-2, IL-13 |
| Th17-type Treg cells | RORγt, STAT3 | CCR4+CXCR3- CCR6+ | IL-17A, IL-17F |
| Th1/17-like Treg cells | T-bet, RORγt, STAT3 | CCR4+ CXCR3+ CCR6+ | IFN-γ, IL-17A |
| Regulatory Tfh cells (Tfr) | TCF1, LEF1, BCL-6 | CXCR5+ ICOS+ PD-1+ |
Table 2: Effector Treg cell subsets and their transcriptional, phenotypic, and cytokine profiles. Generated using data from 1,11.
CD8+ Treg cells
Although CD8+ T cells with suppressive function have been described in mice since the 1970s, so-called CD8+ Treg cells have been less well studied and characterized than their CD4+ counterparts,3,19 particularly in humans. More recent studies have identified CD8+ Treg cells in mice under various inflammatory and disease contexts, including in models of transplantation and cancer.
CD8+ Treg cells arise in the periphery from mature conventional CD8+ T cells. CD8+ Treg cells differ from CD4+ Tregs cells in that most lack FoxP3 expression; instead, these cells have been shown to require the transcription factors Helios and Eomes for their suppressive capacity and homeostatic functions.3,19,20
The most common phenotype used to identify CD8+ Treg cells in mice is CD122highLy49+CD8+ but subsets of CD8+ Treg cells have been shown to express other markers. Surface markers of CD8+ Treg cells include (also see Table 1):3
As with CD4+ Treg cells, none of these markers is unique to CD8+ Treg cells, being expressed on conventional T cells and other immune cell subsets. Proposed subsets of CD8+ T cells and their functions are outlined in Table 3. A recent study identified a population of suppressive CD8+ cells expressing inhibitory killer cell immunoglobulin-like receptors (KIRs) that are increased in people with a variety of autoimmune diseases (as well as COVID-19).19,21 The authors proposed that these cells are the human equivalent of Ly49+CD8+ Treg cells in mice.
| Subset | Precursor | Differentiation condition | Present in young, naïve mice? | Function |
|---|---|---|---|---|
| CD8+FoxP3+ | Mature naïve CD8+ T cells (?) | Alloantigen transplantation | None to low | Suppress effector T cell responses and immune rejection |
| CD8+CD103+ (in vitro) | Mature naïve CD8+ T cells | In vitro TCR stimulation with TGF-β | None | Suppress immune rejection |
| CD8+CD103+ (tumor) | Mature naïve CD8+ T cells | In vivo tumor growth | None | Negatively correlated with tumor control |
| CD8+CD28- | Mature naïve CD8+ T cells | Age-dependent accumulation | None | Suppress effector T cell responses |
| CD8+CD122+CD49d+ | Mature naïve CD8+ T cells | In vivo TCR stimulation | Low | Suppress immune rejection |
| CD8+ CD122+Ly49+ | Unknown | Unknown | Present | Suppress germinal center reaction |
Table 3: CD8+ regulatory T cell subsets in mice. Adapted from 19.
![Flow cytometry - Anti-FOXP3 Antibody [3G3] (APC) (A86268)](https://cdn.antibodies.com/image/catalog/86/A86268_536.jpg?profile=product_image)
![IHC - Anti-CD27 Antibody [LPFS2/4177] (A250621)](https://cdn.antibodies.com/image/catalog/250/A250621_1.jpg?profile=product_image)
![Flow cytometry - Recombinant Anti-CTLA4 Antibody [DM51] - BSA and Azide free (A318645)](https://cdn.antibodies.com/image/catalog/318/A318645_2.jpg?profile=product_image)
![IHC - Anti-CTLA4 Antibody [IHC064] (A324529)](https://cdn.antibodies.com/image/catalog/324/A324529_1.jpg?profile=product_image)
![Flow cytometry - Anti-CD45RO Antibody [UCHL1] (FITC) (A86178)](https://cdn.antibodies.com/image/catalog/86/A86178_481.jpg?profile=product_image)
![IHC - Anti-CD45RO Antibody [T200/797] (A249812)](https://cdn.antibodies.com/image/catalog/249/A249812_1.jpg?profile=product_image)