Ryan Hamnett, PhD | 28th October 2025
B cells orchestrate the humoral arm of the immune system through the production of antibodies, which recognize and bind to foreign pathogens. These versatile lymphocytes are continuously generated within the bone marrow and give rise to a number of specialized subsets. While B cells comprise only about 20% of circulating lymphocytes, they have critical roles in both immediate and long-term immune responses by neutralizing pathogens, orchestrating secondary immune responses, and regulating inflammation, although they also contribute to the pathogenesis of autoimmune and malignant diseases.
For scientific characterization, experimental investigation and immunophenotyping, B cells are commonly identified using pan B cell surface markers. These markers are typically proteins or glycoproteins expressed across most B cell developmental stages, though they may be modulated or lost upon terminal differentiation. Markers of specific B cell subpopulations can be found at the bottom of this page as well as on our Plasma Cell Markers and Memory B Cell Markers, are able to generate a broad repertoire of antibodies to previously encountered infectious agents. After reinfection, pre-existing antigen-reactive clones in the MB cell compartment are positively selected and differentiate into plasma cells. MB cells therefore do not represent a terminally differentiated population.
B cell development is a sequential, multi-stage process that occurs primarily in the bone marrow, where hematopoietic stem cells undergo lineage commitment and a complex process of immunoglobulin gene rearrangement. This enables each B cell to express a uniquely specific B cell receptor (BCR), underlying the adaptive nature of humoral immunity and the ability of B cells to recognize a vast array of antigens. Upon encountering cognate antigens in secondary lymphoid tissues, mature B cells can differentiate into either antibody-secreting plasma cells or long-lived memory B cells, ensuring both immediate protection and the capacity for robust recall responses on pathogen re-exposure.
B Cell Development Markers
Figure 1: Markers of B cell developmental stages
Each developmental stage is characterized by the sequential gain and loss of particular markers (Figure 1). For example, the development in the bone marrow of pro-B into immature B cells involves the acquisition of CD19 and CD20, followed by surface IgM expression, which is a hallmark of immature B cells. Mature naïve B cells in the periphery typically co-express IgM and IgD, and are distinguished by the presence of pan-B markers such as CD19 and CD20, and the absence of activation and memory markers such as CD27.
Activation by antigen results in a cascade of changes, including upregulation of activation (CD69, CD80, CD86, HLA-DR) and costimulatory markers (CD40), as well as a spectrum of differentiation pathways leading to either germinal center reactions (with further maturation and somatic hypermutation), memory B cell formation (characterized by CD27 acquisition), or terminal differentiation to plasma cells (marked by loss of B cell lineage identifiers such as CD19 and CD20, and expression of CD38 and CD138). Details of these pathways and markers can be found on our Plasma Cell Markers and Memory B Cell Markers pages.
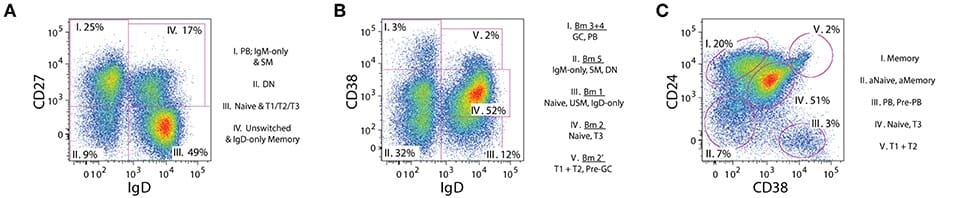
It has been suggested that analysis of the major canonical human B cell subsets can be achieved using 7 core markers, in addition to excluding dead cells and doublets, although several strategies are available to subdivide B cells by flow cytometry (Figure 2).1 Nonetheless, extra markers can assist with further subpopulations or activation states, and more recent, high-dimensional techniques such as mass cytometry and single cell RNAseq continue to discover novel markers and subtypes.2
Figure 2: Widely used flow cytometry gating strategies to isolate B cell subsets. In addition to the markers shown (IgD, CD27, CD38 and CD24), the authors recommend including non-B cell exclusion markers (e.g. CD3, CD14), CD19 as a pan B cell marker, and CD21 to identify activated cells. Edited and reproduced under CC BY 4.0 from 1.
The classification of pan B cell markers refers to proteins broadly expressed across the majority of B cell subsets from progenitor stages through to mature cells, but typically reduced or absent after plasma cell differentiation. Table 1 outlines key pan B cell markers, which are described in more detail below.
| Marker | Expression pattern | Primary use / notes |
|---|---|---|
| CD19 | Expressed from pro B onward; retained on most memory and plasmablasts; downregulated on terminal plasma cells | Lineage gating; broad pan B marker; therapeutic and diagnostic target |
| CD20 | Expressed from pre B through mature B cells; lost during plasmablast to plasma cell transition | Mature B cell identification; therapeutic target for B cell depletion |
| CD24 | High on pre B/transitional B cells; reduced but present on many mature B cells | Developmental staging; distinguishes immature/transitional populations |
| CD45R (B220) | Murine pan B cell isoform expressed from pro B through many mature subsets; human expression variable | Standard murine B cell lineage marker |
| CD72 | Broadly expressed across B lineage up to plasma stages; expression level context dependent | Complementary lineage marker; modulates BCR signaling |
| IgM | Surface from immature B through naive and some memory subsets; absent on pro/pre B and many class switched cells | Isotype and subset discrimination; use with true lineage markers |
CD19
CD19 is the strongest candidate for a true pan B cell marker.3,4 This transmembrane glycoprotein is expressed at the earliest stages of B cell differentiation (Figure 1), from pro-B cells onward, and is retained on most plasma blasts and memory B cells, though it but tends to be significantly downregulated in terminally differentiated plasma cells.
Functionally, CD19 is a member of the immunoglobulin superfamily and a vital signal transduction molecule. It acts as a co-receptor for the BCR complex (often in conjunction with CD21 and CD81), serving to amplify BCR-mediated signaling and lower the threshold for activation.5 This makes CD19 critical not only for B cell activation and proliferation but also for maintaining immune tolerance. CD19 expression is also pivotal in the differentiation and survival of subsets such as marginal zone, follicular, and germinal center B cells. Alterations in CD19 signaling or expression are implicated in immunodeficiency, autoimmunity, and B cell malignancies, and CD19 serves as a diagnostic and therapeutic target in various hematological cancers.6–8
CD20
CD20 is another canonical B cell marker, expressed across developmental stages from pre-B cells up to terminal differentiation into plasma cells.9
As a four-pass transmembrane protein, CD20 participates in signaling cascades linked to the BCR. Although the precise physiological role of CD20 remains incompletely understood, it is known to contribute to B cell activation, possibly by acting as a calcium channel or by modulating BCR signaling in lipid rafts.9 CD20 is also physically associated with HLA-DR and CD40, both important for B and T cell interactions.9
In clinical medicine, CD20 is the prototypical antigen for antibody-mediated B cell depletion (e.g. rituximab and obinutuzumab therapy) in autoimmune diseases and B cell malignancies.9
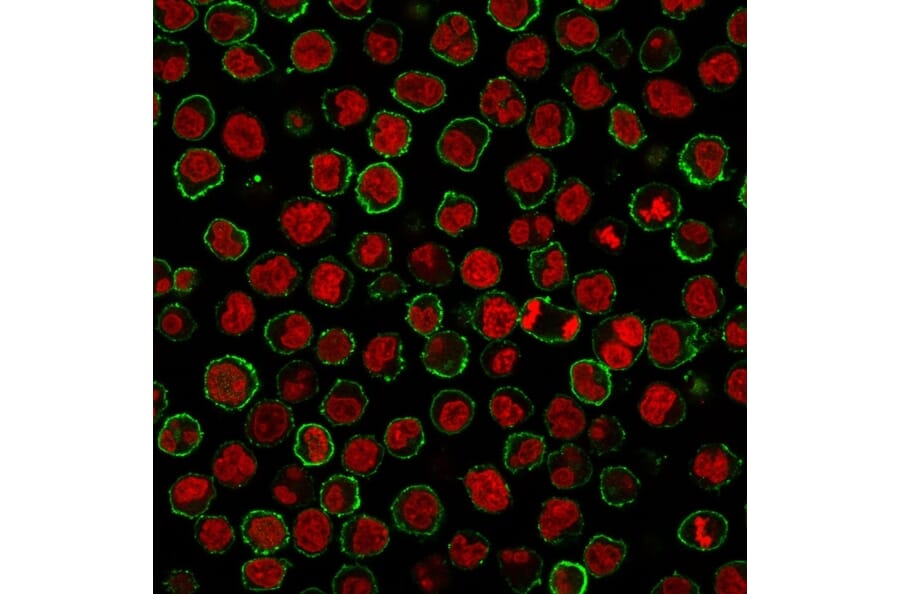
Figure 3: Immunofluorescent analysis of Raji cells withAnti-CD20 Antibody [MS4A1/3409] followed by Goat Anti-Mouse IgG (CF® 488) (Green). Nuclei are stained with RedDot.
CD24
CD24 is a pan B cell marker that is commonly used to distinguish between stages of development due to its variable expression at distinct developmental stages; expression of CD24 peaks at the pre-B cell stage, with continued albeit reduced expression in mature B cells.10 This expression profile reflects its functional significance to B cell maturation, which it regulates through its influence over developing B cell apoptosis.11–13 Because of its broad expression on both solid tumors and blood-based cancers, CD24 has been studied as a potential therapeutic for various cancers.12
CD45R (B220)
CD45R, also known as B220, is a pan B cell marker in mice, expressed from early CD43+ pro-B cells through to plasma cells, while human B cell subsets exhibit more limited expression.14,15 CD45R is a prominent variant of CD45, a transmembrane tyrosine phosphatase that exists as multiple isoforms generated by alternative splicing, many of which are expressed on other immune cell types. In addition to being expressed on B cells, B220 has also been found on dendritic-like cells.15
CD72
CD72 is a co-receptor of the BCR and is found on all B cell developmental stages up to plasma cells. CD100, a protein constitutively expressed on T cells, is the natural ligand of CD72, and can regulate BCR sensitivity by preventing the association of CD72 with the BCR.16 Depending on the context, CD72 can both positively and negatively regulate B cell processes, including proliferation, apoptosis and differentiation.17
IgM
IgM (Immunoglobulin M) is the first antigen receptor isotype expressed during B cell development. Though not a true pan B cell marker given its absence on pro- and pre-B cell stages, it is useful as a marker for immature B cells, transitional B cells, naïve mature B cells, and certain memory B cell subsets.18,19 At the cell surface, IgM constitutes part of the BCR complex, which is essential for antigen recognition and the initiation of signaling cascades that drive cell maturation, selection, and activation. As well as developmental information, the presence of IgM therefore provides functional information on the cell's antigen responsiveness.
B cells can be distinguished into further subsets based on expression of surface markers, localization, developmental history, and function. Among mature B lymphocytes, three broad subtypes are conventionally highlighted: Marginal Zone (MZ) B Cells, Follicular B Cells, and Regulatory B Cells (Bregs). Each subtype exhibits unique immunophenotypic profiles that reflect their specialized functions in immunity, tolerance, and disease. Beyond these subsets, mature B cells can further differentiate into Plasma Cell Markers and Memory B Cell Markers.
Marginal Zone (MZ) B cells reside predominantly in the marginal zone of the spleen at the interface with the circulatory system, where they can quickly respond to blood-borne pathogens.20 Their immunophenotype is shaped by:
Follicular (FO) B cells are the main recirculating B cell subset found in B cell follicles within secondary lymphoid tissues such as spleen, lymph nodes, and tonsils. These cells express:21
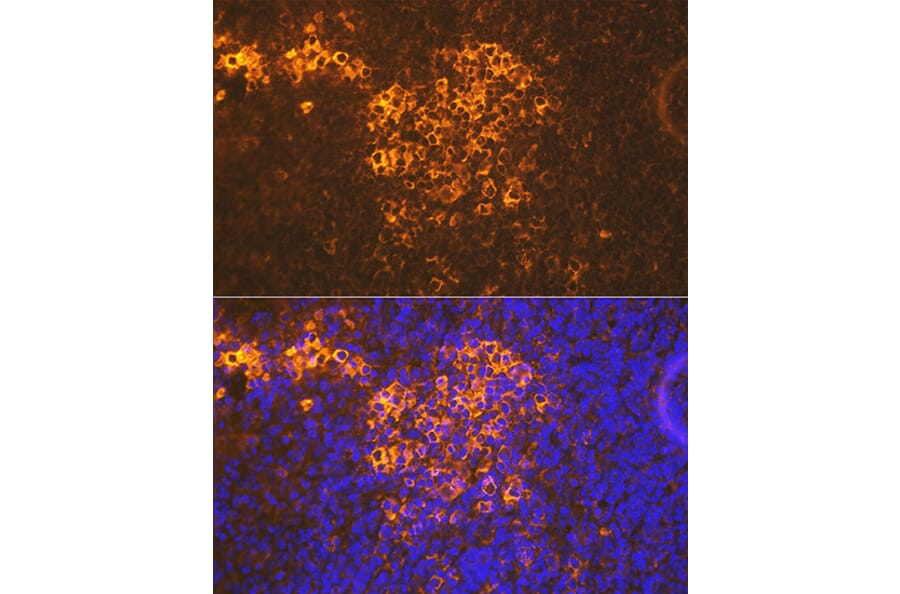
Figure 4: Immunofluorescence analysis of rat spleen using Anti-CXCR5 Antibody [ARC1363] (A308772)at a dilution of 1:100 (40x lens). DAPI was used to stain the cell nuclei (blue).
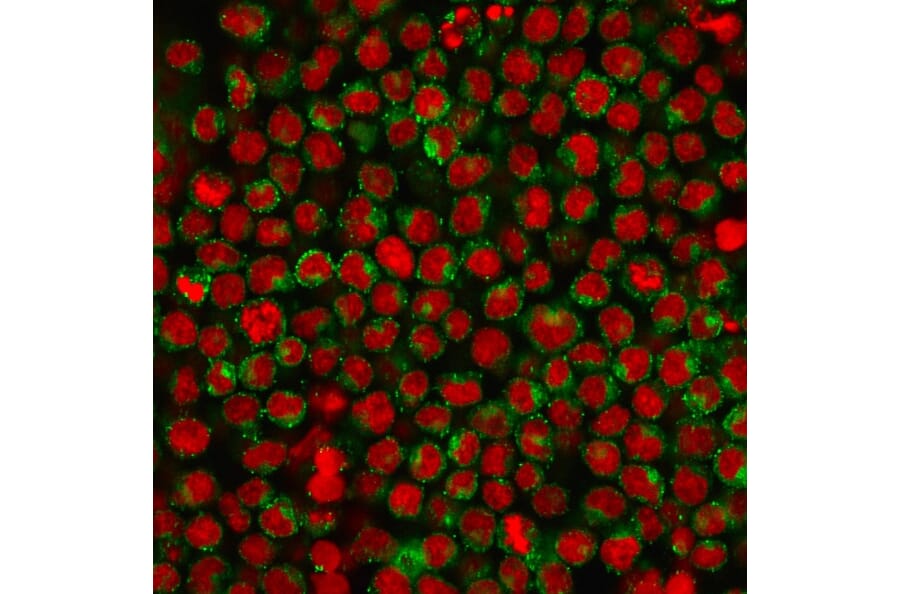
Figure 5: Immunofluorescent analysis of Ramos cells stained with Anti-CD27 Antibody [LPFS2/1611] (A253799) followed by Goat Anti-Mouse IgG (CF® 488) (Green). Nuclei are stained with RedDot.
Regulatory B cells (Bregs) are a less well-defined but important subset distinguished by their immunosuppressive roles comparable to regulatory T cells (Tregs), particularly through the secretion of IL-10, TGF-β, and IL-35.22 They help maintain tolerance and limit inflammation.
Diagrams created with BioRender.com.